You are currently browsing the category archive for the ‘Chemistry Blogs’ category.
On occasion I step off the industrial hamster wheel for a few minutes to have a look around. In Linkedin this morning I saw a post for the 2nd edition of Organic Chemistry by Jonathan Clayden (Author), Nick Greeves (Author), Stuart Warren (Author), Oxford University Press, ISBN-13 : 978-0199270293. From inside the hole along the creek where I spend my free time, I was never aware that Warren had an O-chem textbook.
Amazon allows you to examine a bit of content on-line. If you teach O-chem, this text is worth a look in my estimation.
Many of us are familiar with Warren from his book Organic Synthesis: The Disconnection Approach, 1st edition 1982. A second edition was released in 2008. Retrosynthesis was spreading around to the far-flung corners of the chemistry polygon then. Warren’s book was quite useful in demonstrating that technique for devising an organic synthesis.
An interesting interview of Warren can be found at The Skeptical Chymist from 2009. Warren died in 2021 at age 81.

Just today, as the open door to my golden years stands gaping before me, I learned of a substance called dioxygen difluoride, FOOF. It’s also known as perfluoroperoxide at the NIST Chemistry WebBook site. Seems like I’m always the last one to the oxidizer party. This rather unhappy substance can be prepared as shown below. The word is that the orange-yellow solid is only stable below −160 °C.
O2 + F2 → O2F2 (electric discharge, 183 °C) Wikipedia.
2 O3F2 → O2 + 2 O2F2
Another synthesis can be found in a 1991 paper in the Journal of Fluorine Chemistry.
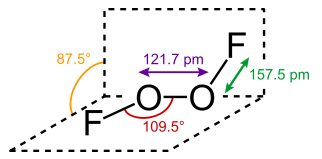
Image from Wikipedia. https://en.wikipedia.org/wiki/Dioxygen_difluoride. A mixture of fluorine and oxygen gas were heated to 700° C then, according to the abstract “rapidly cooled on the outer surface of stainless steel tubes. The tubes were refrigerated by a liquid oxygen bath pressurized to >7600 torr with helium. Six grams of O2F2 were produced in less than an hour.”
Derek Lowe mentioned in one post in his Blog In the Pipeline that FOOF was in the list of materials he won’t work with. Derek also mentioned chlorine trifluoride. A method of preparing this substance is shown below. This substance is a powerful fluorinating agent and reacts in hypergolic fashion with asbestos and sand according to chemist John Drury Clark. Clark wrote a book called Ignition! An Informal History of Liquid Rocket Propellants based on his experiences with rocket propellant research. Clark said that the great toxicity of ClF3 was the “least of its problems”. It’s ability to react in a hypergolic manner with nearly everything was a barrier to its use. It could be stored in metal containers that were first passivated with fluorine gas.
3 F2 + Cl2 → 2 ClF3
Uranium hexafluoride is produced with chloride trifluoride-
U + 3 ClF3 → UF6 + 3 ClF
According to Wikipedia, ClF3 is used to clean Chemical Vapor Deposition (CVD) chambers. Not surprisingly, prior to WWII the Nazis had experimented with ClF3 as a chemical warfare agent called N-Stoff. Production halted when the Red Army overran the facility in 1945. The substance was never used in war.
I had an evil thought just now as I attempt to write 2 reports simultaneously. Why do we keep using that superscripted circle in front of C (i.e., ºC) that designates “degree”?
What the hell? We don’t use it for the Kelvin temperature scale. And, who knows if the engineers use it for Rankine? The thing is useless like an appendix or a titular chairman. Get rid of it!
What do you think?
Today I found myself peering at the lovely lavender glow of opaque argon plasma through the viewing screen of a gleaming new instrument. The light-emitting 8000 K plasma sits apparently still alongside the conical metal skimmer. Somewhere a Dewar was quietly releasing a stream of argon into a steel tube that was bent in crisp military angles into and through walls and across the busy spaces above the suspended ceiling. Another cylinder quietly blows a faint draught of helium into the collision cell. A chiller courses cooled water through the zones heated by the quiet but savage plasma. Inside a turbo pump labors to rush the sparse gases out of the mass analyzer and into the inlet of the rough pump and up the exhaust stack.
Up on the roof, the heavy and invisible argon spills along the cobbles of roofing stones until it rolls off the roof onto the ground where the rabbits scamper and prairie dogs yap. The helium atoms begin their random walk into space. The argon shuffles anonymously into the breeze and becomes part of the weather.
All of the delicate arrangements; all of the contrivances and computer controls in place to tune and play this 21st century marvel. And a wonderment it is. The ICPMS obliterates solutes into a plasma state and then taps a miniscule stream of the heavy incandescent argon breath that trickles into the vacuous electronic salsa dance hall of the quadrapole. All the heat and rhythm for the sake of screening and counting atomic ions. What a exotic artifact of anthropology it is. And it all began in a rift zone in Africa millions of years ago.
The latest rev of Windows 7 and MS office is driving me freaking nuts. Used to be that I could do a graph in Excel and copy it cleanly into Word. That convenience seems to be absent in the latest rev. What fails to copy are the arrows and text boxes that I add to the graph. Not only do some of them fail to transfer, but the graph reformats and they arrive all cattywompus.
What works is to save the Excel document as a pdf and then cut out the graph and paste it into Word. Fancy that.
So, Microsoft, if I could make the dollars I pay for software change form inside your bank account, say, from dollars into Congolese francs, I’d do it this moment.
Here is an interesting question. What fraction of the organic nitrogen in your body is ultimately from the Haber-Bosch Process? Any guesses? This question arose during dinner discussion following a rousing seminar on frustrated Lewis pairs. There is no connection to frustrated Lewis pairs, but the speaker raised the question.
Oh, I don’t have an answer. This happens in science. I’m guessing ~50 %, depending on the extent of protein containing corn products consumed. Any meat science people out there?
Just received a copy of CHETAH 8.0. This is a program for thermochemical and energy release evaluation and is distributed by ASTM. It will calculate enthalpy of combustion and thermochemical properties of compounds and reactions including- LFL, LOC, MIE, lower limit flame temperatures, maximum flame temperature, fundamental burning velocity, and quenching distance.
I have only had it installed for 2 days, so it’s way too early to give an appraisal. It came highly recommended by several colleagues in the process safety field. The only snag so far is a balky SMILES input module. This feature was very appealing because it allows one to copy a ChemDraw structure in SMILES format and paste it into the CHETAH GUI. The rep at ASTM gave me a link which ended up offering very cryptic instructions. Naturally, the problem is some obscure setting in Windows.
Until I get this fixed, I’ll have to enter Benson groups by hand. As it happens, I began studying guitar in my spare time, so there are all kinds of new things for my addled brain to stumble over assimilate. So when I’m not picking at strings, I’m picking at Benson groups.
Update 3/5/09: After a service pack download, the SMILES module is functioning. This is a very powerful tool.
We’ve recently caught up with the times and have been pressing Accelerating Rate Calorimetry (ARC) into service. Or more accurately, paying to have the data collected. ARC is really quite informative in that it can offer a Time to Maximum Rate (TMR) equation from which a TMR can be determined for any desired temperature. You can calculate an adiabatic delta T as well. I do not know how reliable this number is, but it certainly reminds one of the importance of considering the effect of phi factor in process scale up.
The ARC data I get includes an Antoine curve which can indicate that the accelerated rate behavior is or is not characteristic of classical liquid/vapour equilibrium behavior. What this says to the wary is that other volatiles (besides the subject material) may be generated which are not condensable. This is helpful in considering what kind of controllability is available to the process engineers.
A friend who is presently on sabbatical has started a blog about his academic experiences in primarily undergraduate institutions (PUI). It is called Sabbatical Epistles. He mentions a key phrase that is being batted around; it is Transformative Research. According to the NSF, transformative research is-
research that has the capacity to revolutionize existing fields, create new subfields, cause paradigm shifts, support discovery, and lead to radically new technologies.
The context of the use of this phrase was that research funding at PUI’s will increasingly be put to the merit test of transformative research. As such, research into chemical synthesis at PUI’s is especially at risk of not qualifying for funding. I suppose the concern is that multistep synthesis projects for undergrads requires lots of time and skills that undergrads do not have.
Who is against transformative research? It is like motherhood and apple pie. Everybody wants to fund or be part of this kind of effort. We should always ask that research funds be put towards this end. But there is more to it than just an affirmation of meritocracy.
What I sense is that the golden age of undergraduate research programs may be fading into some darker period of scant interest. The scientific establishment continues to grow larger with each passing year. And in parallel, major research universities continue to add programs, courses, grad students, faculty, bricks and mortar, and administration based on the allocation of grant money. Big institutions depend on grant money to a large extent.
As grant money gets tighter, program requirements will increasingly filter the small fish from the big fish. Large institutions have many alumni in influential positions and in the end, the programmatic mind-set of large research institutions in conjunction with the definition of success as understood by administrators of first tier schools will win the day.
There is a pecking order to this. A kind of snobismus. And undergraduate research is not too high in the pecking order. In relation to undergraduate research in the area of synthesis, in most schools this is the only opportunity for an undergrad to get some advanced experience in the synthetic arts. If you have tried to hire a synthetic savvy BA/BS, you know they are hard to find. In my experience, most synthetikkers want to go to grad school. They want more.
Just in case anybody is listening, I want to make a pitch for continued and stronger funding of undergraduate research. As a student, it changed the course of my life in terms of growth and development. As a former mentor of undergraduate researchers as a post doc and prof, I can say that nearly all of my students are now either PhD’s or MD’s. They are all contibuting greatly to the benefit of our society in industry, teaching hospitals, and academia. I am proud of them and I’d do it over in a heartbeat. The pedagogy isn’t in dispute, I suppose. But the method of funding is.
There are as many ways of starting a chemical business as there are people starting them. Entrepreneurs range in profile from smooth talking slicksters to sober, ROI-calculating engineers. Entrepreneurs can also be rather unruly folk. It is not automatically true that business founders are inherently talented at designing and running orgainzations. In fact, they are frequently poor at it. But, successful founders are usually highly focused and are able to attract resources.
A common motivation for starting a business is that the founder is possessed with existential certainty that he/she can operate a business venture better than, say, a former boss or rival. A business founder may be a free spirit, refractory to sensible advice, or may be a solemn Harvard MBA operating by the book. It is not uncommon for a founder to have had several previous failed ventures prior to a successful one.
And make no mistake, the sense of power that a founder feels in the execution of a business plan can be as addictive as heroin or crack. Once a person has had the experience of successfully gathering resources and then allocating them to leverage progress to a goal, they are forever changed. Whether or not they continue the role of managing funds or personnel, their eyes have been opened to the real meaning of power.
Power is the ability to allocate resources.
No matter what kind of chemical business one wishes to start, it is crucial to understand that it will require the accumulation of some kind of resource that you can apply to a problem. That resource can range from your technical reputation, 30 days net of commercial credit, VC monies, or a chemical processing plant. It is all a form of leverage toward the greater goal converting streams of goods and services into streams of cash.
Try to get cash flowing from sales as early as possible. Choosing a Market-Pull activity is the best way to do this.
A chemist starting up a business is able to choose several kinds of general business activities. If you want to be a consultant, you must determine the boundaries of your knowledge and then find demand for that expertise. If you are truly an expert in a field, then more likely than not you know who might buy your services.
If you choose a Technology-Push approach, try to target customers who are willing to be early adopters.
A chemist may be well situated to start an operation offering analytical services. In that case, the enterprising analyst needs to know about underserved demand out in the marketplace. You need to offer a service that prompts people to send a purchase order to you.
If your startup is a one-act pony, it is critical that the pony actually be able to jump through the flaming hoop as advertised. Try to avoid one-act pony business plans. Find Market-Pull products to pay the bills while your Technology-Push products are under development.
A chemist is in a great position to get into formulations. While this might not be strictly a “chemistry’ activity, the walking-around-knowledge chemical that a chemist might have probably well exceeds the basic chemistry knowledge of many “experts” in the formulations business. However, a chemists general knowledge may not be applicable to direct application to formulations. The level of infrastructure for doing formulations can be dramatically less stringent than chemicals manufacturing as well, requiring less startup capital. Again, to be a formulator you need to know what is in demand.
Remember-Sometimes it is dumb to be too smart about things. Be customer oriented. Be honest about strengths and weaknesses. Learn the difference between smart and cagey. Dick was a cagey businessman. Don’t be a Dick.
Fine chemicals manufacture has many success stories. Alfred Bader started his Aldrich empire making what we now call Diazald. Bader was was extremely customer service oriented and I believe this is the key to his success. He visited laboratories asked workers what they needed. If the request was reasonable, he would put the material in the catalog collection. If the chemist-entrepreneur desires to start a catalog fine chemical company to sell reagent chemicals and widgets, then I would advise making a study of that business arena.
Most advisors to entrepreneurs will say that the prospective business person is well advised to put down a written plan. This is important on many levels. The act of writing a business plan is useful to the entrepreneur in several ways. It causes the writer to focus his/her ideas and energy as well as to clarify the goal and how to track towards it. A well written business plan is critical if you need to attract funds to get the operation started. Investors and bankers need a document to study and to bring before others for analysis and buy-in. Just gotta have it.
Starting a drug company is going to be quite difficult for a few isolated chemists to do. It is a complex and insanely expensive and risky business that requires a wide diversity of players to be on board and committed. Somewhere you have to get an MD or MD/PhD, finance people, former pharma executives, regulatory affairs peoples, etc., on the board to add gravitas to your plan. A whole circus of expensive prima donnas. Sounds like a nightmare to me.

Recent Comments