You are currently browsing the category archive for the ‘Chemistry’ category.
A few thoughts on struggle in learning. I’ll confess to having taught undergraduates in the classroom and the research lab environment. My classroom teaching bona fides are limited to 6 years of college level chemistry lecture/lab and quite a bit of one-on-one chemistry tutoring.
Many students approach college chemistry courses with caution. For some, a year of freshman general chemistry is mandatory for their major. Majors such as pre-med, physical therapy, and veterinary medicine require organic chemistry in addition to general chemistry. As my specialty lies in organic chemistry, I have experience teaching both general and organic chemistry students..
From my perspective, general chemistry is as much a mathematics course as it is a science course for many first-year students. A significant portion of general chemistry involves establishing and solving problems that necessitate fundamental algebraic manipulations and calculations. Skills such as balancing equations, maintaining units throughout calculations, and understanding significant figures are essential to master. Additionally, there is the challenge of learning the new vocabulary.
Students who managed to avoid chemistry in high school sometimes found themselves treading water in college chemistry and were afraid of taking two 5 credit hour hits to their GPAs. Most pushed on and got through it. General chemistry is a foundation course and is critical for further pursuits in fields related to the use of chemicals. Unfortunately, a year of gen chem doesn’t really make a person able to function as an independent chemist. It is helpful, though, for technicians in a lab doing routine chemical tests.
Gen chem is to chemistry as The Hobbit is to The Lord of the Rings– it is the necessary prelude to a larger story.
A common problem I encountered while teaching chemistry was the desire of some students to give up hope of ever “getting it”. They would hold off attending office hours to discuss their difficulties until it was too far down the semester timeline. This was usually after a few botched regular exams or a low midterm grade. Frequently the struggling student was having trouble with or neglecting the assigned homework from the text.
Now and then you’d run into a prof who had performance expectations that even they might not have met as an undergrad. They’ll strut around acting as though they were singlehandedly maintaining “proper” academic ideals. Who knows, maybe they had a point. You can try to enthuse everyone using words and pictures, but inevitably there are those who are utterly disinterested, inept or just anxious to put chemistry behind them.
In retrospect, I should have been more direct in calling in more students to office hours who were in grade trouble early in the term. Unfortunately, like many other profs I sometimes subscribed to the sink or swim approach to college education where unsuitable students are culled from the herd. It is a sort of Darwinistic mindset that is easy to fall into. In the end, we have to give all students a fair chance or even a second chance to earn the credentials that the institution confers.
Colleges are organizations that award credentials to verify achievement in meeting or exceeding educational standards set by in-house professors. It tells people that you completed what you started: you navigated a complex maze of intellectual achievements and came out the other side a success.
For any given subject there are always those who struggle with it to some extent. It could be from simple boredom, distractions from real life or the comprehending of difficult material. It may be that the subject just isn’t for them. For myself, I struggled with a foreign language and eventually gave up. I needed full immersion and that wasn’t going to happen. I still regret giving up.
One problem that can often be addressed, however, is the matter of struggle. It seems that many students are not accustomed to struggling with learning. All of us have learned particular subjects successfully because it “just fit” our cognitive abilities, interest or perhaps it was brilliantly presented to us. Or it was a special time in our lives when we were uniquely receptive. It could very well be that previous exposure to the subject was a bit shallow with grade inflation, leading to overestimation of their abilities.
Unfortunately for some, the very necessity of struggle convinces them that the subject is beyond their abilities. They come to believe that if the subject does not immediately stick or appear obvious, then they might as well give up because they will never “get it” along with a collapse of self-esteem.
Giving up on a subject early-on could allow them to switch directions in their education with less time lost and perhaps they would be relieved by that. In this case, giving up is just making a better choice based on experience. Regardless, students should be unburdened early on of the idea that struggle is a predictor of failure. In reality, most learning involves struggle at least to some extent.
Remedies for Struggle
Reading the assigned chapters several times is helpful. First pass, scan the content for a general idea of where the topic is going. A careful reading next with a focus on the example problems is very helpful. Try to understand the example problems and the reasoning presented. Next work on the problem set. If there is time, a third reading can help to cement in the concepts in the chapter. Before going on, work on the assigned problems. Open the solutions manual only if stuck. Struggle with the problem a bit. Success with solving assigned problems can be extremely helpful for a student.
If laboring alone isn’t helping, some schools have tutoring resources available. If not, there are often tutors who will charge on an hourly basis. A few hours of tutoring may be all it takes to get back on track. Sometimes there may be study partners from your class who can study with you. Then again, office hours with your prof or TA can help you over some rough spots. The point is- Struggle!
When I was writing exams, I would look at the example problems in the text as well as the assigned problems. I chose the problems to assign because I felt that they got to the heart of the concepts I held as important to the subject at the level of the content. I would use the assigned problems or those from lecture to write problems using different substances where a reaction would lead to an unambiguous answer. It’s ok to write some questions that require bit of logic to solve, but you can’t turn the exam into an intelligence test.
I once taught a course in chemistry for non-majors. These were students who had tried to get into Geology for Poets or Astronomy but couldn’t get in. They were trapped into taking chemistry for their science requirement for graduation! Early on, a few “representatives” of the class cornered me after a lecture and informed me that “everyone” expected true/false questions on the exams. Pausing, I said I would give them true/false questions, but they would get 1 point for a correct answer, 0 points for no answer, and -1 point for an incorrect answer. The lesson was that if you don’t know something it might be better to just be quiet. After a single exam they never mentioned true/false questions again.
Students eventually realize that chemistry is a highly vertical subject. The more advanced and interesting concepts are built upon or knitted together from those learned earlier. Later coursework will assume that the student has a grasp of content from earlier prerequisite courses. Thirty-one years later the 95 course evaluations from that Catholic women’s college still sit in an unopened envelope in my office.
Find a way to deal with anxiety. Exercise or find a councilor, psychologist, or psychiatrist for help. Anxiety is “druggable”, that is there are meds for it that are very effective. I’m sure there are exceptions, but a family practice doc can’t go very far down the road in treating anxiety. A psychiatrist can fine tune and mix the individual meds to best suit you. It really works.
Most importantly, the student should not EVER get behind in the coursework. It might even be better to drop the class than try to make up for much lost time. The normal rate of chemistry content flow to be absorbed is already high. To have to make up for time lost while also keeping up with the current content flow is often impossible.
Finally, consider that struggle just means that you have to put forth effort to learn. True learning means that your neurons are making new connections in your brain, not just images of something new. To have learned means that your brain has found a way to take diverse inputs and assemble them into part of your consciousness. Sometimes it isn’t easy, but persistence is the key.
Note: What follows are my observations and information from my oncologists and what is scraped off the interwebs. I try to seek information from either primary research literature, medical textbooks or from credible secondary sources. For treatment, I stick to a university medical institution and medical school faculty managing my treatment. I tend not to believe in dietary or nutraceutical approaches. It has been my observation that the origins of cancer are biochemically different from curative or preventative biochemistry. In other words, preventative measures by diet or supplements are mechanistically distinct from the treatment of cancer cells. Divine intervention is not testable, driven by faithful wishing and is supported only by anecdote. I believe that if something truly happens in the universe, it will have an observable mechanism and therefore be measurable.
Oh yes, if you’re squeamish with talking about your prostate because it’s part of your reproductive apparatus, get over it. Part of successfully living with cancer is being able to talk about and learning from it. I’d rather die at least knowing about it.
Because of modern medicine, my experience with both throat and prostate cancer has not been a rocket sled ride to the hereafter. It’s been said that some cancers can be thought of as a treatable, chronic condition and for me that has been true thus far. As luck would have it, my throat cancer was viral in origin and consequently highly treatable by IMRT irradiation and cisplatin. Since 2013 I have had yearly checkups that have all indicated no visible return of the cancer. Since I go to a university medical center, I have had medical students and various head and neck residents also peering down my throat from a camera threaded through my nose picturing my gullet in all of its pink glistening majesty.
The prostate story is a bit different. Before diagnosis the cells had already left the prostate (stage 4) and were judged to be Gleason 9 by histopathology. This was unfortunate. Outside of the prostate capsule they began to wander around through the lymphatic system, lodging in the lymph nodes. Since there was no unified target for surgery or concentrated radiation, The cutters were not called in. Elvis had left the building. After IMRT radiation of the prostate, seminal vesicles and suspected nearby lymph nodes along with 2 years of hormone ablation, my PSA returned to 0.01 ng/mL. Things had taken a turn for the better.
But, the other shoe had to drop eventually. After 9 years, my stage 4 prostate cancer has begun to ramp up steeply. The PSA curve over time (below) is looking more and more like a hockey stick. The borderline PSA value for treatment is 4.00 ng/mL. When it pops up over that value the oncologists begin to take notice. Whether this is based on some statistical mortality data or because of what insurance companies will likely cover is unclear to me. Importantly, PSA may also indicate non-cancerous conditions like prostatitis and benign prostatic hyperplasia. PSA is only an indicator and alone is not definitive. Biopsy is needed to verify and grade the tissue. Of this whole adventure, the biopsy was the worst of it for me. During the procedure, the urologist asked questions about my hobbies -his was carpentry- but I was too distracted to talk about airplanes.
Stage 4 is indicated by histology and backed up by the PET scan revealing radioactive (avid) spots outside of the prostate. Thankfully, this time around nothing was found in the head & neck, chest, prostate or bones. That was good news.
However, the PET/CT scan did show the presence of 5 or so avid lymph glands along the aorta from below the chest to above the prostate.
A proper prostate cancer diagnosis requires more than just a PSA value. An abnormal prostate is detected by digital examination by a urologist and the presence of cancer cells is confirmed by biopsy by a histologist.
But, wait a minute. Exactly what is PSA and what does it do? According to Wikipedia, Prostate Specific Antigen (PSA) is a peptidase enzyme (a protein) secreted by the epithelial cells of the prostate gland. It’s immediate job is to liquify the semen in the seminal coagulum, allowing sperm to swim freely. It is also thought to be involved in dissolving cervical mucus, allowing sperm to enter the uterus. Amounts of PSA above a certain threshold are not normally found in the blood. Elevated PSA is associated with prostate cancer. It’s just a marker.

Serine protease enzymes like PSA have a serine amino acid in the active site of the enzyme which is capable of connecting temporarily with a carbonyl carbon of a (C=O) peptide bond. Since proteins are long chains of peptide bonds, cleaving a peptide bond snips the protein into smaller pieces.
Chemists are all about the mechanisms of chemical transformations and the following has been proposed for a serine protease.

All this said, it turns out that when castration resistance sets in, things begin take a turn for the worse. Prostate cancer cells begin to accumulate in the bone marrow, they begin to interact and develop into tumors that are essentially beyond the reach of treatment. The spine is a common place for them to go, but they can spread to other organs as well.
Of particular interest is the spread of prostate cancer to bone. Prostate cancer cells have an affinity for bone marrow tissue. In my case, the PET/CT scans gave no indication of being present in the head & neck, the chest or bones. That’s good news. In my first round of treatment, I was given 18F-Glucose diagnostic for the PET scan. This time I was given the more receptor-selective 18F PSMA diagnostic called Pylarify. While it is selective for a particular receptor on the cancer cell, it also shows up elsewhere in the body in the PET scan as a result of circulation and transport out of the system. Receptor-specific drugs will bind to the intended receptor, but only after they wander around and stumble into it. This is made less than random due to active transport or solubility partitioning. The effectiveness also benefits by resistance to metabolism and excretion.
Pylarify is a kind of pseudo-peptide containing two modified amino acids, lysine and glutamic acid, joined at the nitrogen atoms as a urea linkage. The key step is the nucleophilic aromatic substitution of trimethylammonium by 18F on the pyridine ring. The presence of abundant heteroatoms (nitrogen and oxygen) groups is not uncommon for pharmaceuticals and is absolutely ordinary for proteins. Heteroatoms serve as hydrogen bond donors and acceptors which is critical in biochemical transformations. A hydrogen bond donor can reversibly bind to a hydrogen bond acceptor and keep the molecules in close proximity long enough for a transformation as well as participate in it.

Pages 29-51, ISSN 0969-8051, https://doi.org/10.1016/j.nucmedbio.2021.12.005.
The interaction of prostate cancer cells in the bone marrow environment is fairly complex and is well described by Zhang X. Interactions between cancer cells and bone microenvironment promote bone metastasis in prostate cancer. Cancer Commun (Lond). 2019 Nov 21;39(1):76. doi: 10.1186/s40880-019-0425-1. PMID: 31753020; PMCID: PMC6873445.
The interaction of prostate cancer cells with bone marrow cells is a topic for another day.
Prologue: What follows is a look at the use of 68Gallium as part of a positron emitting radioligand from an organometallic chemist’s point of view. I’m not from nuclear medicine nor am I a radiation oncologist.
It had to happen … the other shoe has dropped. My stage-4 prostate cancer has come charging back for round 2 after 9 years. Again, I’ve taken a personal interest in radiation oncology. Recently, my PSA shot up steeply through the 4.0 ng/dL threshold triggering an appointment with my radiation oncologist who has ordered a PET/CT scan. Back in 2015 I finished 18 months of hormone ablation (chemical castration) and got the PSA from 29 down to 0.01 with Lupron injections and earlier, a large cumulative dose of x-radiation in the lower abdomen. I have to say that while I experienced no discomfort at all in this round of treatment, I did lose body hair and muscle mass.
PET/CT scanning is an important tool in locating prostate cancer cells. Riding the platform in and out of the scanner is expensive but important. Unfortunately for me, the CT contrast agent is a potent emetic so the scanner becomes an expensive vomitorium ride.
The story of PET, Positron Emission Tomography, has evolved over decades of advancement. To begin, tomography, detectors and computers had to be invented. Separately, positron emission as a medically viable radiation source had to be identified and validated. A substrate for selective delivery of the isotope must be found. In the case of 18Fluorine, it is available as an organofluorine molecule like 18F-Glucose. It turns out that the 18F-Glucose concentrates in clinically useful places and K18F does not.
Positron Emitters
Atomic nuclei that are deficient in neutrons can have an instability leading to emission of a positron (anti-electron with a + charge), also called a β+ decay, which lessens the neutron deficiency by ejecting a positive charge from the nucleus. When a positron is ejected from the nucleus it finds itself immediately swarmed by the electron clouds of surrounding atoms and molecules and doesn’t travel very far. When a positron encounters a negatron (regular electron, β−), they annihilate one another and emit two gamma photons of 511 keV energy at 180 degrees apart. This is a mass to energy conversion. Loss of one positive charge from the nucleus gives rise to a transmutation of the atom causing a one-unit drop in atomic number, that is it goes from n+ to (n – 1)+, but retains most of its atomic weight. In this case, 6831Gallium undergoes positron decay to 6830Zinc.
Positron emitters include 11Carbon (T1⁄2 = 20.4 min), 13nitrogen (T1⁄2 = 10 min), 15oxygen (T1⁄2 = 2 min), 18fluorine (T1⁄2 = 110 min), 64copper, 68gallium, 78bromine, 82rubidium, 86yttrium, 89zirconium, 22sodium, 26aluminium, 40potassium, 83strontium, and 124iodine. This a list given by Wikipedia, but there are many more in more comprehensive tables.
The actual mechanism of β-type emission requires a venture into fundamental particles called quarks. Protons and neutrons are composite particles called hadrons, not fundamental particles. Protons and neutrons are each comprised of 3 quarks, but with a different combination of “up and down flavors” where flavor refers to the species of quark. There are 6 flavors of quarks: up, down, charm, strange, top, and bottom. Interconversion between protons and neutrons can occur if one of the 3 top or bottom quarks changes flavor. By all means, if this interests you, take a dive into it. I shall stop here.
Positron emitters tend to have a short radioactive half-life as well as a limited chemical half-life in the body before they are cleared out through the kidneys or other routes. Ideally, the goal is to deliver a high radiation dose selectively to a target tissue as fast as is safe then disappear. Prolonged irradiation to surrounding tissue is undesirable. The optimal radiopharmaceutical will be highly target selective and have a short half-life. A selective radiopharmaceutical is one that will accumulate in a desired cell type or organ. Accumulation can be aided through simple solubility, the ability to undergo transport through a cell wall, affinity to a specific receptor and the ability to function fast enough to resist the various clearance mechanisms.
A short half-life means that the radioactivity per gram of radioisotope, specific activity in Becquerels per gram, will be at its maximum after activation. Though the radioactivity may be intense, the radiation dose can be controlled by the amount of mass administered. With radioisotopes, there are two kinds of purity to consider: Chemical purity referring to the atoms and molecules present; Radiological purity referring to the presence or absence of other radioactive isotopes. To provide maximum safety and effectiveness, the specific radioisotope with the desired decay mode should be the only source present. If your desired source is an alpha emitter, you don’t need spurious quantities of a gamma emitter present because of inadequate purification.
Economical methods of preparing positron emitters had to be addressed. To fully exploit PET for any given situation, tissue selectivity of radioligands had to be determined and selective positron radiopharmaceuticals developed. Due to the short half-life of these radioisotopes, rapid and safe methodologies to produce them by efficient nuclear transformations, isotope isolation followed by chemical synthesis had to be developed. It is important that isotope generation, isolation and attachment to a ligand be done nearby the hospital for the proper activity to reach the patient.
Positron emitter production involves a nuclear reactor for neutron activation or a cyclotron accelerating protons or deuterons in the preparation. Because both of these sources are highly destructive to organic molecules, an inorganic radioisotope is produced separately and chemically modified to produce an inorganic species that can be chelated or otherwise attached to a radiopharmaceutical. This technique evolved from simple radiography in the 1930’s to a large array of techniques and applications today. The reader is invited to take a dive into this topic.
Since my cancer experience began, a few new radiotherapies and imaging agents have landed in oncology space for prostate cancer. Recently I posted on Pluvicto PSMA (Prostate Specific Membrane Antigen) which was before I knew about my current prostate situation. PSMA is a transmembrane protein present in prostatic cells. Pluvicto uses a chelated 177Lutetium beta emitter as the destructive warhead and a peptidomimetic fragment for binding to the PSMA receptor.
A Brief Interlude into Quality Factor
It should be noted that the various forms of particle (alpha, beta, or neutron) or electromagnetic radiation (x-ray or gamma) have differing abilities to penetrate and cause ionization of within matter. There is a factor for this which is used to refine dosage calculations. It is called the Quality factor, Q.
The destructive effects of radiation stem from its ability to ionize matter along its path. Ionization is a disruptive effect that may result in fragmentation of molecules or crystal lattices into reactive positive or negative ions. Single electron radical species may be formed as well. It is possible for some fraction of the disrupted molecules to recombine if the fragments haven’t already diffused away or gone on to further transformations.
The deleterious effects of radiation on living tissue stems from the amount of disruptive energy transferred to tissues along the path of each particle. Charged particles like electrons, protons and alpha particles tend to dump their energy into matter rapidly and along a short path making them less penetrating than neutrons or electromagnetic rays in general.
Quality factor, Q, is a dimensionless coefficient that is multiplied by an absorbed dose to give a more realistic estimation of radiation energy absorption. Interestingly, the Q for neutrons varies with energy and rises to a maximum around 0.5 to 1 MeV of energy and falls off at higher energies.
The larger the Q factor, the larger the corrected radiation effect. X-, gamma, and beta radiation have a Q factor lower than the others by a factor of 10 to 20. The x- and gamma rays will tend to pass through matter leaving a small amount of their energy to disruption. In radiation therapy this is compensated for by just increasing the fluence or the exposure time.
For clarity, x-rays are generated from the electron cloud around an atom via electron transitions. For instance, if an electron is dislodged from an inner, low energy orbital, another electron can occupy that vacancy by the emission of an x-ray. Gamma rays originate from nuclear energy transitions. Often a nuclear decay might result in a new nucleus that is not at its ground state and would be categorized as metastable. This metastable state, which has its own half-life, can collapse to its ground state by the emission of a gamma ray matching the loss of energy by the nucleus.
Neutrons
Free neutrons are special. They undergo beta decay with a short half-life outside the nucleus having t1/2 = ~ 10-15 minutes, depending on the information source. Not having a charge, they tend to be more penetrating than other particles. However, effective shielding can be had with a hydrocarbon like paraffin or water by virtue of the high concentration of hydrogen nuclei present in these substances. Neutrons are not affected by charge repulsion from an atomic nucleus and therefore can collide and interact with the hydrogen nucleus (a proton). They can scatter from hydrogen nuclei, leaving behind some of their kinetic energy with each collision (see “Neutron Lethargy“). This scattering is the basis for using water to moderate the neutrons in a nuclear reactor. Neutrons are cooled by repeated collisions with hydrogens in water to the point where their kinetic energy of 0.025 eV, which from the Maxwell-Boltzmann distribution corresponds to a temperature of 17 oC, thus the term “thermal neutrons“.
Many elements absorb neutrons, increasing the atomic weight and very often altering the stability of the nucleus leading to a radioactive decay cascade. This is what is happening in neutron activation. In the case of water, the ability of free neutrons to collide with hydrogen nuclei allows them to dislodge hydrogen ions or free radicals from organic and biomolecules resulting in ionization and makes them quite hazardous to living things.
Radioligands
Drugs like Pluvicto are referred to as a radioligand. There is a radioisotope connected to an organic “ligand” for selective binding to a specific protein receptor. A radioligand is injected and diffuses its way a particular receptor where it binds. As it turns out, due to the gamma radiation also emitted by 177Lu, Pluvicto is a radioligand that can also be located in the body by the gamma radiation it emits. In general, a radioligand can be used for two endpoints: To find and signal the location of a particular cell type; and to find and vigorously irradiate a particular cell type.
There are recent radioligand compounds that are used as PET (Positron Emission Tomography) diagnostic agents which selectively bind to the PSMA receptor where they can undergo positron emission revealing the site of prostate cancer cells by tomography. 18F-glucose was first synthesized in 1967 in Czechoslovakia at Charles University by Dr. Josef Pacák and was first tested as a radiotracer by Abass Alavi in 1976 at the University of Pennsylvania on volunteers. Positron tomography came along later. Cancer cells consume glucose faster than normal cells so the 18F will tend to accumulate to a slightly greater extent and reveal their position by positron annihilation. The two 511 keV x-rays simultaneously detected at 180o apart are identified by a ring coincidence detector. A single detection event is discarded.

A radioligand that received FDA approval the same day as Pluvicto was Locametz or Gallium (68Ga) gozetotide. This gallium radioligand targets PSMA as does Pluvicto but is only a PET diagnostic agent.

Locametz has 4 carboxylic acid groups, a urea group and two amide groups aiding water solubility and numerous sites for hydrogen bonding of this radioligand to the receptor. The organic portion of the Locametz is called gozetotide, named “acyclic radiometal chelator N,N’-bis [2-hydroxy-5-(carboxyethyl)-benzyl] ethylenediamine-N,N’-diacetic acid (HBED-CC).” The 68Ga (3+) cation is shown within an octahedral complex with a single hexadentate ligand wrapping around it. The short 68 minute half-life of 68Ga requires that a nuclear pharmacy be nearby to prepare it. The short half-life of 68Ga or other positron emitters as well as the possibility of destructive radiolysis to the ligand prevents preparing a large batch and stocking it. Locametz must be synthesized and transported prior to use. This rules out remote or rural hospitals.
Nuclear Chemistry
So, where does one obtain 68Gallium? Well, there are several methods out there. 68Ge/68Ga generators are produced commercially. One company is GeGantTM who offers 1-4 GBq of 68Ga. (Note: 1 GBq is 1,000,000,000 disintegrations per second).
From the scheme above we see the workings of a 68Ga generator. The ligand attachment is performed exterior to the generator. Atomic nuclei that are neutron deficient like 68Germanium can transform a proton to a neutron. There are two ways this can happen. In Electron Capture (EC) an inner “s” electron can be absorbed by a proton converting it to a neutron and emitting a neutrino by the weak nuclear force. This lowers the atomic number by 1, in this case 6832Germanium becomes 6831Gallium. The other mechanism is for the nucleus to emit a positron (anti-electron) and eject 1 positive charge as a positron (and an antineutrino) from the nucleus, resulting in a new neutron. The atomic weight remains constant, but the atomic number drops by one. If available energy in the nucleus is less than about 1 MeV, an electron capture is more favorable than positron emission.
Once you know about the 68Ge electron capture reaction leading to the 68Ga isotope you have to ask, where does the 68Germanium come from? There are a few different ways to make and concentrate 68Ge and the method you use depends on the equipment available to you. One way is to accelerate protons to a high energy in a cyclotron and slam them into atoms heavier than germanium, such as rubidium or molybdenum. The collision with break the target nuclei into pieces by a process called “spallation“.
Cyclotrons
The first cyclotron was independently invented by Ernest Lawrence 1929-1930 at UC Berkeley. It was the first cyclic particle accelerator built. The idea of the cyclic accelerator was first conceived by German physicist Max Steenbeck in 1927. In 1928-1929 Hungarian physicist Leo Szilard filed patent applications for a linear accelerator, cyclotron, and the betatron for accelerating electrons. Unfortunately for both Steenbeck and Szilard, their ideas were never published or patented so word of the ideas were never made public.
Where does one go to get a cyclotron? One company is Best Cyclotron Systems. If you are not sure of how a cyclotron works, check out the image below from Wikipedia. Note: A cyclotron can only accelerate charged particles like protons, electrons, deuterons and alpha particles which are introduced into the middle of the machine. A key component is the “D” or Dee, so-called because of their D-shape. The cyclotron has two hollow, coplanar Dees which are each connected to a high voltage radiofrequency generator. The Dees are open chamber-shaped electrodes that alternately cycle through positive and negative high voltage attracting and repelling charged particles under the influence of a powerful magnet. Because charged particles change their trajectory under the influence of a magnetic field, the particles follow a curved path of increasing diameter, accelerating until they exit the Dees and careen into the target.

First, the word is out. According to the EIA, the US was the world’s leading oil producer for the 6th straight year in 2023 producing 12.6 million barrels per day.
It is common for people to blame rising US gasoline and diesel prices only on restrictions in crude oil production and alleged government regulatory overreach. Indeed, pressure on the gas and oil supply side or even just the threat of it can lea to unstable retail gasoline and diesel prices. What is less appreciated is the role of petroleum refineries on prices. To be sure, there is always price speculation on both the wholesale and retail sides of gas and diesel pricing to consider no matter the throughput. Like everywhere else, sellers in the petroleum value chain seek to charge as much as they possibly can 24/7/365. Everyone is itching to charge more but are hindered by competition and risk.
Refineries are only one of several bottlenecks in the gasoline and diesel supply chain that can influence retail prices. In principle, more gas and oil can always be produced at the wellhead by increased exploration or increased imports. Even so, there are constraints on transporting crude to refineries. Pipelines have flow rate limitations and storage tank farms and ocean tanker fleets all have finite capacity. Another bottleneck today is access to both the Suez and Panama canals. Suez Canal traffic is threatened by Houthi missile strikes on commercial shipping in the Red Sea and the Panama Canal seems to be drying up. The result is increased shipping costs and delays for international transport which the consumer will have to bear.
What do refineries do?
Refineries are very special places. Within the refinery there is 24/7 continuous flow of large volumes of highly flammable liquids and gases that are subjected to extreme temperatures and pressures for distillation, cracking, alkylates, hydrogenations and reformates. The whole refinery is designed, built and operated to produce the fastest and highest output of the most valuable group of products- fuels. This group would include gasoline, diesel, aviation fuel, and heating oil.
Petrochemicals account for approximately 17 % or refinery output. These petrochemical streams account for pharmaceutical raw materials, polymer products, coatings and films, synthetic fibers, personal hygiene products, synthetic rubber, lubricating grease and oils, paint, cleaning products and more. Regardless of what we may think of plastics and other synthetic materials, the 17 % produced by refineries feeds a very large fraction of the global economy. If plastic bags went away overnight, the whole world would begin to search immediately for alternatives like wood, metal or cotton/wool/flax/hemp.
Occasionally technological challenges confront refineries. An early challenge was the production of high octane anti-knock gasoline. This was investigated thoroughly as early as the 1920’s as the demand for more powerful automotive and aircraft engines was rising. Luckily for the USA, UK, and Germany, the anti-knock problem was solved just prior to WWII. This breakthrough led to aircraft engines with substantially increased power per pound of engine weight.
Leaded Gas
The petroleum that goes into gasoline is naturally rich in a broad range of straight chain hydrocarbon molecules. Straight chain hydrocarbons were used in the early days of happy motoring, but the engine power remained low. While these straight chain hydrocarbons have valuable heat content for combustion, the problem with these molecules is that in a piston engine, they cannot withstand the pressures in the compression stroke that would give greater power. To get maximum power from a gasoline engine, it is desirable to have the piston move up and down as far as possible for maximum power delivery to the crankshaft. However, a long stroke length means greater compression and higher pressure near the top of the compression stroke. Straight chain hydrocarbons could not withstand the higher pressures coming from the compression stroke and would detonate prior to reaching top of the cycle. This effect results in knocking or destructive pre-detonation with power loss.
Tetraethyllead was invented in 1921 by Thomas Midgley, Jr, working at General Motors. After some deadly and dissatisfying work by DuPont, General Motors and Standard Oil Company of New Jersey started the Ethyl Gasoline Corporation in 1924, later called Ethyl Corporation, and began to produce and market tetraethyllead. Within months of startup, the new company was faced with cases of lead poisoning, hallucinations, insanity and fatalities.
The first commercially successful fuel treatment to prevent this pre-detonation was tetraethyllead, (C2H5)4Pb, produced by Ethyl. This is the lead in “leaded” gasoline. The use of (C2H5)4Pb began before WWII and just in time to allow high compression aircraft engines to be built for the war. It allowed for higher powered aircraft engines and higher speeds for the allies which were applied successfully to aerial warfare. The downside of (C2H5)4Pb was the lead pollution it caused. Tetraethyllead is comprised of two chemical features- lead and 4 tetrahedrally arranged ethyl hydrocarbon groups. The purpose of the 4 ethyl groups (C2H5) on (C2H5)4Pb was their ability to give hydrocarbon solubility to a lead atom. It was the lead that was the active feature of (C2H5)4Pb that brought the octane boosting property. At relatively low temperature the ethyl groups would cleave from the lead leaving behind a lead radical, Pb., which would quench the combustion process just enough to allow the compression cycle to complete and the spark plug to ignite the mixture as desired.

While tetraethyllead was especially toxic to children, it was also quite hazardous to (C2H5)4Pb production workers. Its replacement was only a matter of time.

Fuel additives were found that would reduce engine fouling by scavenging the lead as PbCl2 or PbBr2 which would follow the exhaust out of the cylinder. While this was an engineering success, it released volatile lead products into the atmosphere.

Eventually it was found that branched hydrocarbons could effectively inhibit engine knock or pre-detonation and could replace (C2H5)4Pb … which it did. While lead additives have been banned for some time from automotive use, general aviation has been allowed to continue with leaded aviation gas (avgas) in light piston engine aircraft like 100 octane low lead (100LL). Only recently has leaded avgas become a matter of public concern.
A refinery not only engineers the production of fuel components, it must also formulate blends for their customers, the gas stations, to sell. The formulations will vary with the season and the location. Some gasolines have ethanol, other oxygenates like MTBE, octane boosters, detergents and more. One parameter is the volatility of the fuel. When injected into the cylinder, it must evaporate at some optimum rate for best fuel efficiency. This will depend on the vapor pressures of the components.
Back to Refineries
The production volumes of the individual fuel products will not match the contents of the crude oil input. Gasoline is the most valuable product, but more gasoline leaves the refinery than arrives in the crude. Any given grade of gasoline has many, many components and the bulk of them have somewhere around 8 carbon atoms in the hydrocarbon chain. Wouldn’t it be nice if longer hydrocarbon chains could be broken into smaller chains to be added into the gasoline mix? And guess what, that is done by a process called “cracking”. A piece of equipment called a “cat cracker” uses a solid ceramic catalyst through which hot hydrocarbon gases pass and get cut into smaller fragments.
But what about straight chain hydrocarbon molecules? Wouldn’t it be nice to “reform” them into better and higher octane automotive fuels? There is a process that uses a “reformer” to rearrange hydrocarbon fuels to give better performance. The products from this process are called reformates.
Reforming is a process that produces branched, higher-octane hydrocarbons for inclusion in gasoline product. Happily, it turns out that gasoline with branched hydrocarbons are able to resist pre-detonation and have come to replace tetraethyllead in automotive fuels entirely. Today we still refer to this lead free gasoline product as “unleaded”.
Octane and Cetane Ratings
Octane rating is a measure of resistance to pre-detonation and is determined quantitatively by a single-cylinder variable compression ratio test engine. Several octane rating systems are in use. RON, the Research Octane Number, is based on the comparison of a test fuel with a blend of standard hydrocarbons. The MON system, Motor Octane Number, covers a broader range of conditions than the RON method. It uses preheated fuel, variable ignition timing and higher engine rpm than RON.
Some gasoline is rated in the (R + M)/2 method which is the just average of the RON and MON values.
In both the RON and MON systems, the straight chain hydrocarbon standards are n-heptane which is given an octane rating of 0 and the branched hydrocarbon 2,2,4-trimethylpentane, or isooctane, which is given an octane rating of 100.
Tetraethyllead and branched hydrocarbons are octane boosters. Methyl tert-Butyl Ether (MTBE), ethyl tert-butyl ether, and aromatics like toluene are also used to boost octane values. Internal combustion engines are built to use a gasoline with a minimum octane rating for efficient operation. A rating of 85 or 87 are often the octane ratings of common “unleaded” gasoline. Higher compression ratio engines require higher octane fuel- premium grade -to avoid knocking.
For comparison, diesel has a RON rating of 15-25 octane so it is entirely unsuitable for gasoline engines. Diesel has its own system called the Cetane rating. The Cetane Number is an indicator of the combustion speed of the diesel and the compression needed for ignition. Diesel engines use compression for ignition unlike gasoline engines which use a spark. Cetane is n-hexadecane which is a 16-carbon straight chain with no branching. Cetane is given a Cetane Number (CN) of 100. Similar to the Octane rating, the branched 16-carbon hydrocarbon heptamethylnonane, or isocetane, is given a CN of 15. Included in the Cetane number.
Refineries must keep close tabs on seasonal demand for their various cetane and octane-rated products as well as the composition of the crude oil inputs which can vary. Each gasoline product stream has performance specifications for each grade. While gasoline is a refined product free from water, most sulfur and solid contaminants, it is not chemically pure. It is a product that contains a large variety of individual hydrocarbon components varying by chain length, branching, linear vs cyclic, saturated vs unsaturated members that together afford the desired properties.
Specific Energy Content
Absent ethanol, the combustion energy values of the various hydrocarbon grades are so similar as to be negligeable. The energy content of pure ethanol is about 33 % lower than gasoline. Any energy differences would be due to subtle differences in blending to achieve the desired octane rating or proprietary additives like detergents. A vehicle designed to run on 85 octane will not receive a significant boost in power with 95 octane unless it is designed to operate on higher octane fuel.

From the Table above and looking at the polypropylene (PP) and polyethylene (PE) entries then comparing to gasoline, we see that the specific energies are the same. The two polymers and gasoline are saturated, hydrocarbons so it is no wonder they have the same specific energies. Polystyrene is a bit lower in specific energy because the hydrogen content is lower, reducing the amount of exothermic H2O formation as it burns. The point is that by throwing away millions of tons of PP or PE every year, we are throwing away a whopping amount of potential fuel for combustion and electrical energy generation.
Petroleum based liquid fuels burn readily because of their high vapor pressure and low flash points. Polyolefins like PP and PE by contrast have virtually no vapor pressure at room temperature and consequently are difficult to ignite. In order to burn, polyolefins need to be thermally cracked to small volatile fragments in order to provide enough combustible vapor for sustained combustion. Plastic fires tend to have an awful smell and dark smoke because the flame does a poor job of energizing further decomposition to vapor.
Going from E10 to E85, the specific energy density drops considerably from 43.54 to 33.1 MegaJoules per kilogram (MJ/kg). Replacing a significant quantity of gasoline with the already partially oxidized ethanol lowers the potential energy. In the tan colored section, we can see the elements silicon to sodium. These elements are either very oxophilic or electropositive and release considerable heat when oxidizing. Some metals amount to a very compact source of readily oxidizable electrons.
Refinery Troubles
According to the US Energy Information Agency (EIA) US refinery output in the first quarter of 2024 has dropped overall by 11 % and has fallen as low as 81 % utilization. Decreasing inventories are causing rising retail prices. Still, average gasoline and diesel prices are currently below the same time period in 2023.
According to EIA, the US Gulf Coast has seen the largest 4-week average drop in refinery utilization at 14 % since January, 2024. This is attributed in part to the early start of maintenance shutdowns of Motiva Port Arthur and Marathon Galveston Bay refineries which account for 7 % of US capacity.
Weather has factored-in this year as refinery production was halted in several locations in the US. A severe winter storm shut down the TotalEnergies’ 238,000 barrel-per-day refinery in Port Arthur, Texas.
Oil production in North Dakota fell to half. Oil production was estimated to have fallen between 600,000 and 650,000 barrels per day.
Exxon Mobil Corp returned a fluidic catalytic cracker and a coker to normal operation at its 564,440 barrel per day refinery in Baytown, Texas.
A Flint Hills Resources 343,000 barrel per day refinery in Corpus Christi, Texas, was significantly impacted by unseasonably cold weather including freezing rain.
The largest refinery in the Midwest, BP’s 435,000 barrel per day refinery in Whiting, Indiana, was taken off-line by a power outage and forced a 10 % drop in refinery utilization in the Midwest the first week in January. Normally the Midwest region produces as much gasoline and diesel as it consumes. This rich local supply leads to somewhat lower prices in the region.
As I bumble and tumble through the chemical literature I frequently run into interesting chemicals and chemistry. Today’s moment of chemistry is with the “Wine Lactone”, so called because it is found in, well, wine. Interestingly it was first identified in koala urine. I saw that this was an opportunity also to dissect the chemical name of the Wine Lactone and perhaps answer questions that you didn’t know you had.
There are numerous forms of the wine lactone that have seemingly minor differences but have different odors. Some of the other “forms” are called stereoisomers and others positional isomers. The atomic composition is the same, but the atoms and their bonds are arranged in a slightly different way. It is not uncommon for these differences to result in a change to the odor or some other property.
The problem with chemical names (nomenclature) for people outside of chemistry is that they seem to be over-complicated polysyllabic tongue twisters with numbers and sometimes Greek letters that are impossible to pronounce or remember. Indeed, they are very often complex and seem to have a mysterious origin. This is where chemistry has strayed away from medieval naming “habits” and supplanted it with a systematic naming system that describes the exact atomic composition, how the atoms are connected and, if necessary, the particular shape in three dimensions.
For thoroughness I’ll point out the molecular formula style like CxHyNzOt where x y, z and t are variable numbers. Other elements were left out for convenient description here. Any organic molecule can be described by the numbers of carbon, hydrogen, nitrogen, oxygen and other atoms present. While the molecular formula is an accurate representation and is necessary for calculating molecular weight, as a unique identifier it is not very useful. Any given polyatomic molecule may have more than one structure that fits the molecular formula.
There are several groups that have been influential in chemical databases and nomenclature around the world. German chemists were on top of this early on with the German language Beilstein database and system of nomenclature (1881) for organic substances, now maintained by Elsevier Information Systems in Frankfurt. For inorganic and organometallic substances, there is the Gmelin database (1817) which is maintained by Elsevier MDL.
The systematic nomenclatures I will be referring to are IUPAC (International Union of Pure and Applied Chemistry) and CAS (Chemical Abstracts Service) supported by the American Chemical Society. I am unaware of the volume of usage of Beilstein and Gmelin databases today. They appear to be ongoing. Not being a German speaker, I’ll use first CAS then IUPAC in that order of priority. CAS and the few other databases use a numbering system for each unique substance in addition to the name. The CAS registry number, CASRN, is used around the world for authoritative identification of chemical substances. This includes academic R&D, industry, Safety Data Sheets, transportation, emergency response and not just in the USA. CAS also manages the TSCA registry list for EPA.

Many chemicals have names that pre-date systematic modern naming conventions like toluol or methylbenzol (methylbenzene, toluene) or vinegar acid (acetic or ethanoic acid). These older, trivial names are deeply entrenched in common usage and the secret cabal of nomenclature mandarins lets it pass uncontested.
Above is a ball and stick 3-D model of the Wine Lactone and next to it is a diagram of the numbering system for the molecule. While any fool could number the atoms, it takes a special one to make it official. The heading of the graphic gives the IUPAC name of the lactone as done by a chemical graphics application called ChemSketch. For comparison, the CAS name is given as well. The CAS database entry for the structure gives a very slightly different version of the same thing.
R&S designations can be omitted if they are not known. Adding R&S to the structure gives a spatially accurate view. It is not uncommon for a structure to be disclosed and given a CASRN before any R or S features are known.
The starting point for assigning a name is to decide what the core structure is, noodle through its numbering and then begin identifying the fragments on it. Somebody in the murky depths of time determined that the core structure of the Wine Lactone is a variety of 5-membered ring called a “furanone” (FYUR an own). The C=O (carbonyl, CAR bun eel) part could be in two places so we’ll have to account for that. With non-carbon atoms in the ring, the non-carbon atom is usually given the place number of “1”.
Both CAS and IUPAC have publications on organic ring structures, however in my experience IUPAC does not show the numbering scheme as CAS would. CAS holds a list of all known ring systems.
Question: Why doesn’t sophomore organic chemistry teach CAS nomenclature rather than IUPAC. Answer: I don’t know other than IUPAC has been taught for a good long time and is usually limited to fairly simple molecules in class. I suspect that the professor’s background as well as the textbook content are involved.
Before we go on, we notice that a hexagonal 6-membered ring is attached at two adjacent places to the 5-membered ring. This is a “ring fusion” and fused 6-membered rings are often given the radical “benzo”. So, the core structure is a type of “benzofuranone”. Oh yes, here a radical is a word fragment added to a name to indicate the presence of something.
Starting with oxygen at position 1 we go around the edge of the fused ring skeleton clockwise and attach numbers to the carbon atoms that are not part of the ring fusion. In the graphic above you can see that there were ring atoms that received simple digits. The atoms that make up the fusion are named by taking the number of the atom that precedes it and adding the character “a” to it.
So, what do we know already? We have a benzofuranone with C=O (carbonyl) at position 2. The “one” radical of furanone indicates that the furan ring has a carbonyl group in it.
Next we must account for the way in which the molecule is arranged in 3-dimensions. Carbon atoms need to have 4 bonds (lines) connected to them. If all of the lines are single, the carbon has 4 atoms arranged around it in the shape of a tetrahedron with the attached atoms at the 4 vertices. A wedged line means that the atom at the end is jutting up and out of the plane of the page. Dashed lines indicate that the group on the end is jutting down below the plane of the page, but the artistic license here is that the dases are omitted. Notice that there are 3 wedged lines at positions 3, 3a and7a. The two hydrogen atoms (H) are projecting up out of the page as is the CH3 (methyl) group. This tells us that the two rings are jutting behind the page, so this molecule is not flat but bent. The name of the molecule has to indicate this.

The carbon atoms at 3, 3a, and 7a are called stereocenters because they have molecular handedness. Note that each is connected to four different groups in the molecule. It sounds like crazy talk but it is quite important. We won’t burrow into details here. Suffice it to say that these atoms will have an extra letter to designate what kind of “handedness” they have. R is for rectus meaning right-handed and S is for sinister meaning left-handed. There are rules for determining R vs S which we will not go into here.
Handedness in a molecule isn’t important except in how they interact with other molecules with handedness. The two nonsuperimposable (chiral) mirror images are said to be “enantiomers” (eh NAN tee oh mers). This is an issue for crystal structure and for many biomolecules. Outside of this, it isn’t much of a concern.
We now have (3S, 3aS, 7aR) to be plopped into the name. This group is shown in parentheses.
Next, we tackle the “tetrahydro” radical- it indicates 4 more hydrogen atoms are present than what would otherwise not be there. In nomenclature they start with rings that are unsaturated in hydrogen, meaning that the carbon skeleton is not connected to as many hydrogen atoms as it could. The four positions where a single hydrogen has appeared are 3a, 4, 5, 7a on what would otherwise be double bonds. There is one more to account for. The namesake furan molecule would have a double bond at position 3. In this molecule there is a hydrogen atom in place of the double bond, so 3H is added with the CH3 group.
So far we have (3S, 3aS, 7aR) and 3a, 4, 5, 7a-tetrahydro and 2-benzofuranone.
At positions 3 and 6 there are two CH3 or methyl groups. To account for position and the fact there are two of them leads to this part of the name- “3,6-dimethyl-“. Elsewhere in the name we denote the R or S configuration, if any. The CH3 at carbon 6 is flat so it lies in the plane pf the page- it is neither R nor S. But the CH3 at carbon 3 juts out of the page at us rather then pointing downward. It has been given the S configuration.
Putting it all together in the CAS name, the configurations at relevant atoms are given first followed by a hyphen then the hydrogen locations followed by a hyphen then the word “tetrahydro”. After tetrahydro radical and a hyphen, the methyl positions 3,6 are added followed by a hyphen then radical “di” attached to the radical “methyl” followed by a hyphen then the core structure 2(3H)-Benzofuranone. The “2(3H)” feature indicates that the carbonyl is at position 2 and an H is at position 3, indicating that the furan ring is connected by single bonds.
I describe here the name of the Wine Lactone in its extended CAS form rather than the parsed form. If you want to sort numbered chemical names alphabetically, leading digits just complicate the sorting. So if you sort alphabetically by the core structure, you rearrange the name to lead with Benzofuranone followed by the details trailing off in the distance as in the first graphic.
(3S,3aS,7aR)-3a,4,5,7a-tetrahydro-3,6-dimethyl-2(3H)-benzofuranone.
I’m sure that deep within the lower catacombs at Chemical Abstracts in Columbus, OH, there are grizzled old nomenclature wizards who may quibble with my explanations, but let them materialize before me in a puff of smoke and discuss the error of my ways.
Prologue: I want to give my bona fides on appreciation of the “US space program.” For as long as I can remember I have been a space enthusiast. I followed projects Mercury, Gemini, Apollo, Skylab, X-15, Space Shuttle, ISS, Voyager’s 1 & 2, Cassini and others in real time. Even though space publicists mention scientific research, they never go into more than the very least they can get away with for fear of MEGO- My Eyes Glaze Over. To its credit NASA posts annual lists of research papers with links disclosing research results from R&D conducted in the orbital environment. Here is such a list. Much of the research might seem arcane but it is important to realize that the practical value is likely to come later as others incorporate it into their subsequent research and product development. This is how R&D works.
A few words about Elon Musk’s plans on moving mankind to Mars. As everyone knows, Musk is actively engaged in developing space craft large enough, numerous enough and powerful enough to take a great many people to Mars. His stated dream for humanity is to transport a large number of people to the red planet to establish a permanent settlement- a sort of Earth 2.0 for humans. There is even fanciful talk of terraforming Mars for more convenient and safer occupation. This is a colossal job, even for a small world like Mars.
All energy produced and consumed on Mars will be electrical via nuclear energy, solar, or maybe wind (??) generation. Combustion as we know it is out due to the absence of combustible materials and abundant oxygen. Solar power generation will be limited by reduced solar energy shining on Mars and by the practical problem of dust accumulation. Thermoelectric generation from a Radioisotope Thermoelectric Generator (RTG) has been the solution used on many Mars landers and deep space probes.
The best radioisotopes for RTG are alpha emitters. Alpha particles are +2 charged helium nuclei which cause a large amount of ionization over a short distance as it crams its way through matter, stopping in a short distance. Because they lose energy over short distances even in air, alphas require very little shielding, unlike beta and especially gamma radiation.
Betas themselves are easily shielded, but as they decelerate in matter, they can generate radiation called braking radiation, or bremsstrahlung x-rays, which are more penetrating. This is how x-rays are generated in an x-ray tube. Electrons impacting a target like copper generates x-rays. The effect is more pronounced in higher atomic number (high Z) elements like copper, but in low Z materials like plexiglass x-ray generation is much reduced. Consequently, beta emitters are commonly shielded with plexiglass.
The main downside to RTG is the low efficiency in converting thermal energy to electrical energy via the Seebeck effect– about 3-5 % currently according to most sources. So, for every 100 watts of thermal energy production, only 3-5 watts of electrical energy are available. This puts pressure on the supply of scarce radioisotopes.
On the good side of RTGs, they are stable, reliable and long lasting. Waste heat can be used to provide warmth for proper operating temperature in the craft or facility. The Mars lander Curiosity uses 4.8 kg of 238PuO2 to produce 100 watts of electrical power.
The deal with the devil you have to make with RTG power generation is that the best heat generating isotopes in terms of power density (watts/g) also have the shortest half-lives. For instance, 210-Po has a high power density of 140 watts/g but a half-life of only 0.38 years. It undergoes a 5.6 MeV alpha decay directly to stable 206-Pb, emitting a gamma only once in 100,000 alpha decays. Gamma emission poses shielding weight penalties and radiation hazards both in manufacture and operation in space. Even with no humans around, there is still the matter of electronic components that are sensitive to radiation. The more commonly used alpha emitter 238-Pu has a lower power density of 0.54 watts/g but a reasonably lengthy half-life of 87.7 years and minimal shielding requirements.
The background radiation environment in space by itself demands that shielding and radiation hardened electronics be used. Any added radiation from an on-board RTG only compounds the problem. The amount of shielding any given material provides is measured in half-thickness, not “full thickness” and is dependent on the type and energy of the particle. This value is the thickness of a specific material required to reduce the intensity to half of the incident radiation, not the total radiation emerging from the shielding material. This is because scattering can occur within the shielding material contributing to or minimizing the total flux. The point of this is that shielding only attenuates radiation to acceptable levels and not to zero.
238-Pu is a synthetic isotope that must be isolated from other Pu isotopes as well as a dog’s lunch of other elements in spent nuclear fuel or be selectively synthesized by nuclear chemistry. Isotopic separation of 238-Pu from other plutonium isotopes is difficult, slow and not the preferred method of producing it at scale. Nuclear chemistry that provides exclusively 238-Pu from a single transformation as with like 237-Np, offers a more productive route. This allows good old regular, valence-electron chemistry to effect the separation needed.

238-Pu is produced by neutron irradiation of 237-Np producing transient 238-Np with its 2-day half-life and subsequent beta decay to the 238-Pu. Chemical separation of the plutonium from residual neptunium is straightforward but, like all chemistry with radioisotopes, burdened by the need for radiation shielding for safety.
238-Pu is presently in short supply in the US. The Savannah River Site was producing “bulk” 238-Pu but was shut down in 1988. After closing of Savannah, the US purchased 238-Pu from Russia but the word is that Russia is short on it as well. In recent years other sites have been scaling up production where “scaling up” means producing in the several hundred grams to a few kilograms in a campaign.

In the RTG, plutonium is not used in the metallic state but as the oxide which is a ceramic or refractory** material like most heavy metal oxides. The plutonium is oxidized to 238PuO2, pelletized and clad in corrosion resistant iridium. According to NASA, this refractory form of plutonium is resistant to an accidental release in a variety of accident scenarios including Earth reentry and rocket propellant fires.

The Seebeck effect is not the only means of producing electrical energy from radioactive decay heat. The free piston Stirling Radioisotope Generator can use decay heat to drive a piston in a Stirling engine using helium gas as the working fluid. Waste heat is dumped at the cooled end of the engine and the linear reciprocating motion of the free piston is used to generate electrical power in the adjacent alternator.
The electric alternator is similar to the electromagnetic flashlight on the market. It works on the ordinary induction principle buy moving a magnet through a coil. You shake the flashlight to recharge it, causing the internal magnet to move back and forth through a coil. Shake it for 1 minute to get 4 minutes of light. The Stirling radioisotope free piston linear alternator operating in this manner can produce 4 times the electrical power of an RGT.

In 2020 workers Wong and Wilson at the NASA Glenn Research Center reported that they were able to operate a Stirling radioisotope power convertor for 14 years maintenance-free.
Off we go!
Some thought will be needed on screening potential migrants to Mars for age, various physical ailments, dental health, genetic predispositions, sociability and underlying psychological issues. A manic crew member could drive fellow crew members to a murderous rage over time. Such screening has been done with astronauts for a long time. I wonder if choosing to migrate to Mars isn’t a sign of some precarious psychological condition in itself, after all the likelihood of a return to Earth may be slim. It would resemble going to jail in some ways.
Over time, the masses of new Martians living in Muskville will have to decide on what to do with themselves beyond exploratory geology, meteorology and engineering studies of Martian accommodations. Mars is a big, arid and frigid desert with no breathable air. But it may offer a few choices for recreation such as spacesuit hiking and shuffleboard. The outdoor choices will be limited by the Muskvillager’s battery, heating and oxygen supplies as well as ability to get around.
Eventually, all manner of psychological, social and physical maladies will manifest in Muskville and will have to be dealt with. People will spontaneously form cliques eventually giving us-vs-them issues requiring mediation. Unless the New Martian settlers are sterilized, pregnancy is a near certainty. An entire book could be written on complications this would bring. The alternative is to limit the inhabitants to a single gender or to gay individuals- most likely a non-starter.
Death on Mars means that your remains will need to rest somewhere outside the facility. A fresh body will freeze stiff in the Martian cold and remain that way indefinitely. Digging a grave will require energy expenditure and digging tools. Cremation will consume considerable power and may be out of reach.
Something like a hospital with medical supplies and trained staff will have to be present. The few physicians who might be present will be required to be generalists with exceptional diagnostic and surgical skills. A full medicine cabinet to cover a range of maladies will be needed to support this.
As Muskvillagers age out, the range of health problems will widen and require care. Diabetes, cancer, dementia etc. will fade in and people will age and die. This will leave job openings and duties behind which will have to be filled.
In general, the conveniences of modern living will be seriously cut short for the New Martians for a long time. A supply line with Earth that can withstand politics, business failure and war must be maintained.
If I were planning a migration to Mars, I’d worry about maintenance and spare parts for everything. Mechanical things will break. Perhaps an orange-colored Home Depot module will hitched to the back of the lander and sent along with a load of duct tape, assorted bolts and screws, sealant, O-rings, hand tools and cleaning supplies. Don’t forget a few bags of peanut M&Ms.
Wherein I jump to conclusions.
The human capacity for folly knows no bound. Woven in with folly are variable education, emotional inputs and diverse belief systems. The migrants will carry religious and political predispositions that they may or may not reveal in screening for candidates. Friends and relatives on Earth will sicken, age and eventually die but access to a return trip to earth may be severely restricted or effectively impossible.
On reflection, establishing even a modest Mars base will involve large energy inputs. Getting to the surface of Mars with enough reserve propellant for the return trip, the establishment of shelter, oxygen and water supplies are the priorities. Beyond just surviving day-to-day, there is interest in the possibility of putting Martian minerals to use as building materials or even water and oxygen production.
There are indications of frozen water on the surface of Mars in certain limited locations. Where there is water there is the possibility of using electric power to produce oxygen. The hydrogen produced may have utility somewhere but its use for combustion seems unlikely due to the corresponding amount of oxygen needed.
Anywhere you have silicates, aluminates and metal oxides, you have oxygen. Silicon and aluminum both have a strong affinity for oxygen and as such represent a thermodynamic well requiring steep energy inputs for oxygen extraction from minerals. Even worse, many silicates and aluminates are oligomers, chain polymers or network polymers that render them insoluble solids with high melting points. Silicates, aluminates and metal oxides are all comprised of a central atom- silicon, aluminum, or a metal -that are electron deficient by virtue of being connected to oxygen anions. In order to liberate oxide from oxidized silicon, aluminum or a metal, something negatively charged needs to come in and displace the oxide species. Metal oxides like the iron oxides are very often refractory requiring high temperatures to react. Then there is a long list of oxyanions like sulfate, phosphate, hydroxide, chromate, ferrates, molybdates, titanates, tungstates, manganates, etc., each with metal cations. After these there are the polyoxyanions …
The point is that there are a wide variety of oxide species to be found in rock and soil with differing properties. In the end, a negatively charged oxide anion must be oxidized to produce molecular oxygen.
A thermodynamic well resides in a substance where atoms come together to form strong chemical bonds and release a great deal of heat into the surroundings. The same amount of energy that was released is minimally what would be needed to drive the reaction in the reverse direction. This bond forming heat energy is dispersed amongst the large number of surrounding molecules. The heat evolved in forming the original bond produces a high temperature locally, but as it spreads out each succeeding layer of neighboring molecules gets a smaller and smaller share of the original energy. As the bond forming energy release is spread over more and more molecules, the resulting temperature rise of the succeeding layers get smaller and smaller.
Newton’s Law of Cooling says that rate of heat flow is directly proportional to the temperature difference between contacting objects. The greater the temperature difference, the more useful work (heat and work are both energy) that can be done. Large temperature differences transfer large quantities of energy. Low temperature differences result in less energy transferred. As the heat spreads to the surroundings it produces decreasing temperatures as the heat conducts away and the ability to do work diminishes. This constrains the recovery of waste heat.
The diffusion of energy in this manner is what entropy is about- the irreversible loss of energy to the surroundings. If you are tempted to talk about entropy, consider that it has to be consistent with its unit of measure: Entropy, S, equals energy per degree Kelvin or Joules/Kelvin (J/K).
In order to get molecular oxygen from minerals it will require a great deal of energy expenditure per kilogram of oxygen. Not only that but specialized equipment and chemicals. Any oxygen produced will have to purified and compressed into cylinders.
MOXIE
The extraction of molecular oxygen from the abundant carbon dioxide atmosphere seems desirable and has actually been put to the test on Mars. A prototype molecular oxygen generator called MOXIE went to Mars on the Perseverance rover and successfully produced oxygen from carbon dioxide beginning in 2021.

The MOXIE oxygen generator is a solid oxide electrolysis device that operates at 800 oC and uses a stack of scandia stabilized zirconia ceramic electrolyte. An excellent source of information on MOXIE can be found at this Science site.
About 10 % yttria (Y2O3) or scandia (Sc2O3) will prevent the zirconia (Zr2O4) electrolyte from undergoing a phase change that causes the ceramic to fail at high temperature. From personal experience I know that scandia is chosen as a better diluent for zirconia because it allows lower temperature operation than yttria by perhaps 200 oC. The lower operating temperature with scandia allows for better sealing of the cell. High temperature seals are very problematic at these operating temperatures.
The MOXIE electrolysis cell uses a nickel coated cathode for reduction of the CO2, a ceramic zirconia/scandia electrolyte that allows oxygen anions to selectively pass through, and an anode where the anions are oxidized and combine to form O2 where it is captured. MOXIE produced O2 at a rate of 6-8 g/hr while on Mars. The process vents carbon monoxide waste as well as unreacted CO2 at the cathode where it is vented.
A limiting factor in operating MOXIE is the operating voltage across the cathode and anode. Two kinds of chemistry can occur within MOXIE. Carbon dioxide can be reduced to form oxide or carbon, depending on the flow rate of CO2 and the operating voltage. The Nernst voltage, VN, is the minimum voltage necessary to do the chemistry. At about 1.1 volts the cell will reduce CO waste biproduct to carbon on the cathode. This is called “coking”. Carbon formation on the cathode impedes the function of the cathode and reduces the output of the cell. The voltage for coking varies very little with flow rate.
The VN for the desired reduction of CO2 to oxide (O–) and CO at a low flow rate is around 1.0 volts and at high flow rates drops to about 0.95 volts or just a bit lower. So, the “normal” operating voltage range then would be between 1.0 and 1.1 volts to prevent fouling the cathode with coke. The operating voltage window seems a bit narrow. It was found that while a stable operating voltage could be supplied, the resistance of the cell was very sensitive to temperature making stable operation a bit delicate.
Pyrochemistry
Extraction of oxygen from lunar mineral samples has been done previously (below). All of the mineral samples were iron rich and gave yields of 2 to 5 % in the form of water. The samples were from Apollo 17 and consisted of ilmenite (FeTiO3), basalt, soil and volcanic glass. The process uses hydrogen at a reaction temperature of 1050 oC producing H2O. Presumably the water vapor is mixed with hydrogen during and after the reaction. The water can be isolated by simple condensation in the presence of the hydrogen.
Reduction of Ilmenite: FeTiO3 + H2 — > Fe + TiO2 + H2O

by Carlton C. Allen
Lockheed Martin Engineering and Sciences Co.
To use the process described above, high temperature is required for the hydrogen reduction in a refractory vessel. This requires considerable electrical energy input to heat the thermal mass of the vessel and the regolith. Spent material will have to be removed and discarded. Perhaps the heat can be recovered for general facility heating. Oh yes, the recovered water will need to be electrolyzed to produce molecular oxygen and hydrogen. This process will use plenty of electric power as well as for the compressors to store the O2 in pressure bottles. In principle the hydrogen can be recovered for reuse in the hydrogenation vessel.
The above process applied to ilmenite produces metallic iron and titanium dioxide, a white pigment. FYI, ilmenite is a common raw material for high purity titanium dioxide production. It is high purity because the titanium dioxide is prepared from titanium tetrachloride which is isolated by distillation from the ore matrix after fluidized bed chlorination.
The first Martian settlers will have to bring every single thing necessary to live on the planet. That includes launching it and landing it on the surface intact. Landing on Mars is tricky because the atmosphere is too thin to provide much aerobraking. The Martian surface pressure is the same as the Earth’s at 80,000 ft altitude and the temperatures are frigid.
Let’s say we successfully land a crew and set up housekeeping. What are they going to do with their time? These missions are supposed to last about 2 years including a lengthy transit time. They can collect various kinds of data on Martian geology and weather and send it back to earth. Somebody will get publications out of it. Eventually, somebody will decide that there must be other things to do besides geology and meteorology. Naturally there will be much ongoing R&D on the pragmatics of living on a remote Martian outpost in a crowded pressure can.
Eventually, the question of what non-research living will look like. Shelter will need construction from some kind of materials. Every new section of shelter will need to be airtight and equipped with environmental controls, sanitation and power. Bulkheads between sections will need to be in place to isolate calamities.
Support staff will be needed one day to provide critical services and perform facilities maintenance. This would also include medical staff, emergency care, food & sanitary support, electronics and IT support and administrative staff for the inevitable paperwork. The lander will need rocket engineers for upkeep and repairs to assure launch reliability for the return trip. Do rockets exist that can sit for a year fueled and then reliably launch and insert into a trajectory back to Earth? There are many, many problems to be resolved in many areas.
After some period of time, a crime will happen on Mars. It could be petty theft, assault or even murder. Someone will have to be appointed to look after law and order. An astronaut-sheriff, sergeant at arms or just the po-leese. What kind of due process will be available to a suspect in a Martian colony? Guns will be too risky to have in the settlement given that a bullet could pass right through a bad guy and rip through the structure creating a leak.
On earth, doing independent research requires getting academic credentials, finding a position, grading exams for goddammed freshman chemistry, executing an R&D program, and then going home every day to refresh and have a social life. Imbedded in all of this we have courtships, marriage, mortgages, babies and divorce. We manage the ten thousand details of modern life and interact with our families and social networks. We mourn those we lose and celebrate our achievements. We enjoy good health and suffer injury and sickness and eventual death.
On Mars, the equation will be a bit different. Many of the above life elements will apply, but from a great distance. Instead, we will be confined to a small space with an unchanging group of fellow crew members. The distance to Earth from Mars is constantly changing and there will be a period absent any communication when the earth is behind the sun.
Eventually, research on living in space or on Mars will wind down to minutae if it hasn’t already and people will have to find other things to do. The funding for living off-world will have to switch from R&D to … what, a lifestyle?
I wonder if there will ever be room for commerce and jobs on Mars. I can see running a canteen or restaurant for profit but stocking them with earth supplies will be prohibitively expensive and infrequent.
What joy can there be living in a pressure can on a hostile planet? What few hermit-astronauts there may be might find it acceptable if they never need a dentist. Perhaps dentures or implants should be routinely fitted to all visitors to Mars.
The second stage of Mars exploration will have to ramp up progress on sustainability. Using Martian soil as raw materials for construction and for crops. As the Martian population rises beyond the first few rotating crews, what will the immigrants do with their time in can-living on a hostile world? Would going to Mars to lead an utterly confined life with nothing to do be an attractive draw?
Epilog
I think that settling on Mars is not such a great idea overall and specifically would be wasteful of resources that should be applied to the rehabilitation of the biosphere on our home world. It would somewhat resemble living on the Amundson-Scott Station on the south pole but without the benefits of breathable air or supplies regularly shipped in. Further, the lack of radiation shielding on the surface of Mars will offer 40-50 times the background radiation as on Earth, not counting the occasional storm of angry solar protons the sun flings out now and then.
** NASA does not use the terms “ceramic or refractory” in its description of the 238-Pu heat source. This is my choice of words.
As wondrous as our physical and chemical senses are, they are severely constrained in a few fundamental ways. Our vision is limited to our retinal response to a narrow, 1-octave wide band of electromagnetic radiation. As it happens, this band of light can be absorbed non-destructively by or stimulate change in the outer, valence level of inorganic and organic molecules. Electrons can be promoted to higher energy levels and in doing so temporarily store potential energy which can then do work on features at the molecular level. In the retina, this stimulates a polarization wave that propagates along the nervous system.
Owing to the constraints of the optics of the band of light we can sense, we cannot see atoms or molecules with the naked eye. This is because the wavelengths in the narrow range of visible light are larger than objects at the atomic scale. Instead, we perceive matter as a continuous mass of material with no indication of atomic scale structures. No void can be seen between the nucleus and the electrons. For the overwhelming majority of human history, we had no notion of atoms and molecules.
Democritus (ca 460-370 BCE) famously asserted that there exist only atoms and vacuum, everything else is opinion. The link provides more detail. The point is that atoms and vacuum were proposed more than 2000 years ago in Greece. The words of Democritus have survived over time but I’ll hazard a guess that the words were not influential in the rise of modern atomic theory in the 19th and 20th centuries. A good question for another day.
In all chemistry, energy is added to the valence level of a molecule as electronic, rotational, vibrational or translational energy.
Thumbnail Sketch of the Interaction of Light and Matter
Radio waves are a band of long wavelength that can interact with electrically conductive materials. Electromagnetic waves having a wavelength greater than 1 meter are considered to be radio waves. As a radio wave encounters a conductor, the oscillating electric field of the wave causes charge to oscillate in the conductor and at a rate matching the radio wave. Radio waves, whether in electronic devices or in space, are formed by the acceleration of charged particles. Recall that when you cause a charged particle to change it’s direction of motion, e.g., by a magnetic field, it is undergoing an acceleration. It is useful to know that radio waves are non-ionizing.
Microwave energy causes dipolar molecules to rotate back and forth by torsion as the waves pass. This rotational energy can be transferred to translational and vibrational energy through collisions, raising the temperature. The molecule does not need fully separated charges like a zwitterion, but molecules may have less than full charge on one side and a less than a full opposite charge on the other side, like water. This is a dipole. Water has a strong dipole and is susceptible to absorbing energy from microwaves.
Infrared radiation causes individual chemical bonds and entire frameworks to vibrate in specific ways. The Wikipedia link for this topic is quite good. When a molecule absorbs heat energy, it is partitioned into a variety of vibrational modes which can bleed off into other energy modes, raising the temperature.
Ultraviolet light is energetic enough to break chemical bonds into a pair of “radicals”- single valence electron species. These radicals are exceedingly reactive over their very short lifetime and may or may not collapse back into the original bond. Instead they can diffuse away and react with features that are not normally reactive, leading to the alteration of other molecules. UV light is very disruptive to biomolecules.
X-rays are more energetic than ultraviolet light and can cause destructive ionization of molecules along their path. They can dislodge inner electrons leaving an inner shell vacancy. An outer shell electron can collapse into the inner vacancy and release energy that can eject a valence level electron, called an Auger electron. This alters the atom by ionization and giving a change in reactivity. X-rays are also produced by the deceleration of electrons against a solid like copper though lighter targets can also produce x-rays.
Gamma radiation originates from atomic nuclei and their energy transitions. They are the highest energy form of electromagnetic radiation and cover a broad range of energies at <0.01 nanometer wavelengths. Many radioactive elements emit only gamma rays as a result of their nuclei being in an unstable state. Some nuclei can emit an alpha or beta particle resulting in an unstable nucleus that will then emit a gamma to relax.
The wavelengths of radio waves are too long and too weak to interact with biomolecules. Some radio waves come from the synchrotron effect where charged particles like electrons will corkscrew around magnetic field lines of a planet and release energy in the form of radio waves. In the case of Jupiter and it’s moon Io, a stream moving charged particles are accelerated by a magnetic field, the particles will emit mainly in the 10 to 40 MHz (decametric) range of radio waves as they spiral around the magnetic field lines into Jupiter. Jupiter’s volcanic moon Io sends charged particles into the planet’s polar regions where the magnetic field lines bunch up. This leaves a visible trace of borealis-like gas that glows. That radiation is emitted in the shape of a conical surface. It is only detectable here when the cone sweeps past earth as Io obits Jupiter.

NASA/GSFC/Jay Friedlander

An atomic nucleus can absorb or emit gamma rays. For instance the gamma emitter Antimony-124 emits a 1.7 MeV gamma that can be absorbed by Beryllium-9 which photodisintegrates into a 24 kiloelectron volt neutron and two stable He-4 nuclei. This nuclear reaction can be used for surveying for beryllium ore deposits by detecting neutron backscatter.
The purpose of this cooks tour of the electromagnetic spectrum is to suggest that visible light band is uniquely suited to interact with matter just enough to induce valence-level chemical changes. The change can be charge separation or an alteration in the composition or shape of a molecule. The changes may be reversible or not.
Ok, done with that.
So, not all electromagnetic radiation plays nicely or at all with any given chemical substance. The narrow visible band of light is uniquely well suited to interact non-destructively, mostly, with living things. Chemistry is about the behavior of the outer, valence level of electrons around and between atoms and molecules.
The retinas in our eyes send signals to the brain continuously that result in a very curious thing- our perception of color registers instead of just a grey scale. Not just the colors of the rainbow, but also more nuanced perceptions like pastels, brown and in their many textures- all with binocular vision!
The constraints on human vision depend on the chemical composition and anatomical structures of the retina as well as the construction of the brain. As the description of the various bands of electromagnetic radiation suggest, there is much to the universe that our senses cannot detect. We do not directly view the radio, microwave, infrared, ultraviolet, x-ray or gamma ray views of the universe.
Our daily understanding of the universe is mostly framed by what we can see with the unique biochemistry and anatomy of the retina. It’s not a bad thing with its limitations, but for an appreciation of the true scope of the universe we would have to find ways to view in the other electromagnetic radiation bands. And, we do. With radio telescopes and satellites that pickup x-ray and UV energy to give images. Now with JWST, we’re peering deeper into the universe as revealed by infrared energy. The longer wavelengths of infrared can pass through clouds of dust particles that previously blocked our view in the optical spectrum.
The structures of the atom and molecules are characterized by the very large fraction of “empty” space they contain. Electrons seem to be point charges with no measurable size. Yet they have mass, spin and the same magnitude of charge but opposite that of the much heavier proton. And, the proton is not even a fundamental particle but a composite particle. It’s like a bag with three hard objects in it.
The universe is wildly different from what our senses present to us. All matter1 is made of mostly empty space. What we see as color doesn’t exist outside of our brains. Our sensation of smell is the same. Cold is not a thing. It is just the absence of heat energy. Finally, our consciousness exists only in our brains. It is a natural phenomenon that is highly confined, self-aware and may be imaged through its electrical activity or F-19 MRI with fluorinated tracers. This wondrous thing is happening on the pale blue dot floating in the vastness of empty space. So far, we can’t find anywhere else in the observable universe where this occurs.
It is good to remember that we search for extraterrestrial intelligence to a large extent with radio telescopes. On earth, the use of radio communication is a very recent thing, tracing back to the beginning of radio in 1886 in the laboratory of Professor Heinrich Rudolf Hertz at the University of Karlsruhe. Hertz would generate a spark and find that another spark would occur separately.
By 1894, Marconi was working on his scheme to produce wireless transmissions over long distances. The wider development of radio transmissions/receiving is well documented, and the reader can find a rabbit hole into its history here.
In order for the discovery of radio transmission to occur, several other things must have been developed first. The discovery of electricity had to precede the development of devices to generate stable sources of electricity on demand and with sufficient power. Then there is the matter of DC vs AC. Some minimal awareness of Coulombs, voltage, current, electromagnetism, conductors and insulators, and wire manufacturing is necessary to build induction coils for spark generation.
James Clerk Maxwell had developed a series of equations before the discovery of wireless transmission by Hertz. Hertz was very familiar with the work of Maxwell from his PhD studies and post doc under Kirchhoff and Helmholtz. Hertz was well prepared in regard to the theory of electromagnetism and was asking the right questions that guided his experimental work.
Radio transmission came to be after a period of study and experimentation by people like Marconi, Tesla and many others who had curiosity, resources and drive to advance the technology. As the field of electronics grew, so did the field of radio transmission. It’s not enough to build a transmitter- a receiver was required as well. Transmitter power and receiver sensitivity were the pragmatics of the day.
This was how we did it on earth. It was facilitated by the combined use of our brains, limbs, opposable thumbs and grasping hands. Also, an interest in novelty and ingenuity during this period of the industrial revolution was popular. While people who lived 10,000 years ago could certainly have pulled it off as well as we did, the knowledge base necessary for even dreaming up the concepts was not present and wouldn’t be for thousands of years. The material science, mathematics, understanding of physics, and maybe even cultures that prized curiosity and invention were not yet in place.
In order for extraterrestrials reaching out to send radio signals that Earthlings could detect, they would have to develop enough technology to broadcast (and receive) powerful radio transmissions. If you consider every single mechanical and electrical component necessary for this, each will have had to result from a long line of previous developmental work. Materials of construction like electrical conductors could only arise from the previous development of mining, smelting and refining as a prelude to conductor fabrication to produce a way of moving electrical current around.
Radio transmission requires electrical power generation and at least some distribution. None of this could have been in place without the necessary materials of construction, mechanical and electrical components already in place. Most of the materials would have to have been mined and smelted previously. Electrical power generators need to be energized by something else to provide electricity. On earth we use coal or natural gas to produce steam that drives generator turbines to make electricity. Also, there is nuclear and hydroelectric power. ETs would face a similar problem for the generation of electrical power.
If you follow the timeline leading to every single component of an operating radio transmitter, you’ll see that it requires the application of other technologies and materials. It seems as though a radio transmission from extraterrestrial home planets need something like an industrial base to get started.
What if there were intelligent extraterrestrials who were not anatomically suited to constructing radio transmitters for their own Search for Extraterrestrial Intelligence or just for local use? Perhaps they are +very intelligent but not far along enough yet to have developed radio. Or, what if they were just disinterested in radio? What if they used radio for a short window in time and have been using something else not detectable from earth, like what we do with optical cable? The point is that we would never hear them by radio, yet they would be there.
Surely there is a non-zero probability of this happening. This dearth of signal may be so prevalent that we will conclude that we are alone in our local region of space. Perhaps funding will be cut and we’ll quit looking. We can take that finding to fuel our sadness of being alone in the cosmos. Or we could use it to appreciate just how unique life is and take better care of ourselves.
Instead of buying into Elon Musk’s vision of colonizing Mars and somehow terraforming it, why don’t we terraform Earth back into a place where the biosphere is more stable and pollution is pushed back to some lower level? A utopian vision for sure, but it also has a non-zero probability of happening.
1. Not including dark matter, if it really exists. I remain skeptical.
A recent raid on a clandestine drug lab in the Hatzic Valley east of Vancouver, BC, netted 25 kg of “pure” fentanyl and 3 kg which had already been cut for street use. Precursor chemicals used to manufacture the fentanyl were also seized. Along with the drug, the raid also seized 2,000 liters of chemicals and 6,000 liters (about 30 drums) of hazardous chemical waste, according to an RCMP news release 2 November, 2023.
The police said that the seizure represented 2,500,000 street doses.
In August of 2023 the police in Hamilton, Ontario, announced the results of Project Odeon. This was a large-scale sweep of illicit drug production in the Hamilton and Toronto area. From January 1, to July 30, 2023 there were 606 incidents related to suspected opioid overdoses and 89 suspected drug related deaths in the Hamilton area. Twelve people were charged for a total of 48 criminal charges. The police disclosed the following items that they seized-
- An operational fentanyl drug lab at 6800 Sixteen Road, Smithville.
- A dismantled fentanyl drug lab at 4057 Bethesda Road, Stouffville.
- Approximately 3.5 tons of chemical byproduct from fentanyl production.
- 800 gallons of chemicals commonly used in the production of fentanyl
- Lab equipment commonly used in the production of fentanyl
- 64.1 kg of illicit drugs, including 25.6 kg of fentanyl, 18 kg methamphetamine, 6 kg of ketamine
- A loaded, Glock firearm and ammunition and four extended magazines
- Over $350,000 of seized proceeds, including cars, jewelry, furniture and cash
Fentanyl is a synthetic drug first prepared in 1959 in Belgium by Paul Janssen (1926-2003). Janssen was the founder of Janssen Pharmaceuticals, now a subsidiary of Johnson & Johnson. In addition to fentanyl, the Jenssen team developed haloperidol, the ultrapotent carfentanil, and other piperidine based congeners. Piperidine itself is a DEA List 1 substance in the US.
The elephant in the room with fentanyl is its extraordinary potency as an opioid. In pharmacology, potency is a quantitative measure of the amount of dose needed to elicit a specific effect on an animal or human in terms of dose weight per kilogram of body mass. Potency is subject to variability across a population and rises to an asymptote which can be difficult to pin down. For these reasons potency is reported at 50 %. For highly potent drugs like fentanyl, the measure is expressed as milligrams or micrograms of dose per kilogram body weight (mg/kg or mcg/kg body weight). One milligram per kilogram is one part per million (ppm).
When matters of toxicity arise, it is important to remember the maxim that “the dose makes the poison”. This observation traces back to Paracelsus in the mid-sixteenth century.
“All things are poison, and nothing is without poison; the dosage alone makes it so a thing is not a poison.”
– Paracelsus, 1538. Source: Wikipedia
Fentanyl acts much like morphine in regard to its affinity for one particular opioid receptor. Morphine is commonly the “standard” with which other opioids are compared. For instance, fentanyl is said to be 50-100 times more potent than morphine. Only 0.1 mg of fentanyl is equivalent to 10 mg of morphine. Carfentanil is more potent still at 10,000 times the potency of morphine.
Morphine is an agonist which activates the μ-opioid receptor. Activation of this receptor with morphine produces analgesia, sedation, euphoria, decreased respiration and decreased bowel motility leading to the earthly delights of constipation. Fentanyl is thought to interact with this receptor as well.

So, how is fentanyl synthesized? See the synthetic scheme above. I’ll just comment on the Janssen synthesis and some issues. I have no idea of how it is made out in there by the Mexican cartels and in ramshackle American trailer parks. The synthesis above has some steps that may be undesirable for backwoods or jungle operations like hydrogenation. In the first step, aniline will be needed to make the phenyl imine. It’s pretty toxic and stinks to high heaven. Next, lithium aluminum hydride is needed to reduce the imine double bond to an amine. This innocent looking grey powder is very hazardous and should only be used by an experienced chemist. It is also available as a solution in tetrahydrofuran. The next step is the formation of the amide with propionic anhydride. While the reaction entails a simple reflux, you still have to isolate the product. Once you have recovered the amide, the benzyl protecting group on the piperidine nitrogen must be removed. It allowed amide formation exclusively on the upper aniline nitrogen and has served its purpose. Finally, the piperidine nitrogen must be festooned with a phenylethyl group and phenylethyl chloride was used to afford the fentanyl product.
An excellent review of the pharmacology and drug design of this family of opioids, see Future Med Chem. 2014 Mar; 6(4): 385–412.
In chemical synthesis generally, substances are prepared in a stepwise manner and with as few high yielding steps as possible. To begin, one must devise a synthesis beginning with commercially available raw materials as close to the target as possible. If the product has many fragments hanging off the core structure, it’s best to solve that problem early. Synthetic chemistry is almost always performed in a non-interfering solvent that will dissolve the reactants and allow the necessary reaction to occur. A low boiling point is preferable for ease of distillation. An important side benefit from a solvent is that it will absorb much of the heat of reaction which can be considerable. Left on its own, a reaction might take its solvent to the boiling point by self-heating, generating pressure and vapor. The benefit from evaporation or reflux boiling is that as a solvent transitions from liquid to vapor there is a strong cooling effect which helps to control the temperature. An overhead condenser will return cooled solvent to prevent solvent loss.
You can do any chemical synthesis in one step with the right starting materials. Unfortunately, this option is rarely available. The next best option is to take commercially available starting materials through a known synthetic scheme. People who run illicit drug labs are never interested in R&D. They want (and need) simple chemistry that can be done by non-chemists in buckets or coke bottles at remote locations. Chemical glassware can be purchased but sometimes the authorities will be notified of a suspicious order. This is especially true with 12 liter round bottom flasks.
The most difficult and risky trick to illicit drug synthesis is obtaining starting materials like piperidine compounds in the case of fentanyl and its congeners. In the case of heroin, acetic anhydride shipments have been investigated for a long time because it is used to convert morphine to heroin- an unusually simple one-step conversion. Solvent diethyl ether is similarly difficult to get outside of established companies or universities. Many other common drug starting materials are difficult to obtain legally in the US or EU by the criminal element. However, China is thought to be a major supplier of starting materials outside the US and EU. Countries with remote coastlines, loose borders, lackadaisical or corrupt law enforcement reduce the barriers for entry of drug precursors. China in particular has a large number of chemical plants that make diverse precursors for legitimate drugs. Unfortunately, some of these precursors can also be used for illicit drugs or existing technology adapted for this use. Precursors can be sold to resellers who can do as they please with them. Agents may represent many manufacturers and can mask the manufacturer’s identity and take charge of the distribution abroad. Shady transactions become difficult for authorities to detect and trace. The identity of illicit precursor chemicals are easily altered in the paperwork to grease the skids through customs. Resellers can repackage chemicals to suitable scale, change the paperwork and jack up the price for export. It has been my experience that many if not most Chinese or Japanese chemical manufacturers conduct business through independent export agents. However, behind the curtains there often a byzantine web of connections between companies and agents, so you may never know who will manufacture your chemical. As an aside, this complicates getting technical information from the manufacturer since the agent will not disclose a contact at that manufacturer.
Highly potent drugs like fentanyl must be taken in very small dosages which means that kilo-scale batch quantities of drug result in many individual sales per kilo. Small quantities of highly potent drugs are more easily smuggled than bulky drugs like weed with its strong odor.
There is a down-side to the illicit manufacture of drugs like fentanyl. It is quite toxic at very low dosages and must be handled with the greatest of care lest the “cook” and other handlers get inadvertently and mortally poisoned. Good housekeeping helps, but I have yet to see a photo of a tidy drug lab.
Fentanyl can be sold as a single drug but perhaps is cut with a solid diluent that some random yayhoo decided was Ok to use. Other drugs of abuse like heroin may be surreptitiously spiked with fentanyl to kick up the potency. In either case, a given dosage may or may not be safe even for a single use. There is no way for a user to know. Also, the concentration or homogeneity of mixed solids may be subject to wide variation. For more than a few people, their first fentanyl dose will be their last.
[Reissue under better title]
Due to a recent hospital stay with pneumonia, I found myself staggeringly bored. To stave off some of this I began to look into an antibiotic I was given that I had never heard of- Levofloxacin. The structure of this antibiotic was different from antibiotics I was previously familiar with. Natural I suppose, considering that I’ve been immersed in organo-transition metal chemistry for most of my industrial career. Metal-carbon bonds are quite useful in some sectors but not as drugs.
Levofloxacin is a good place to go deep diving into some of the murkier depths of chemical nomenclature. The complicated-looking chemical naming system exists to unambiguously represent the composition and shape of molecules. Certain features and properties of a molecule confer important attributes that need categorizing, thus requiring descriptive names rather than just a number. Every different chemical substance is, well, different and their chemical names must reveal a unique identity. Two or more substances with the same name leads to nothing but trouble.
Chemical substances can be grouped into categories to associate them with related aspects. We have noble gases, transition metals, hydrocarbons, pnictogens, polymers, acids, and bases etc. But the categories allow for variation when particular attributes are under discussion.
The names of chemical substances can be very off-putting to non-chemists and often does lead them to abandon their search for information. A few have even suggested that if you cannot pronounce the name it must be bad. Even worse than the polysyllabic and numbered character strings are the various synonyms. Consider simple toluene which is actually not so bad-
In chemical nomenclature there is just a bit of flexibility in how numbers, syllables and name fragments can be assembled as the toluene example above shows, if you don’t read the rules too closely. The plethora of names come from historical trade names or long-time industrial use or may just predate systematic nomenclature now in use. There is also the German Beilstein and Gmelin organic and inorganic nomenclature as well, but these seem to be outdated.
As always, a proper chemical name describes the composition and 3-dimensional connectivity of the chemical structure of a molecule. These names are commonly listed in one of the two dominant styles of chemical nomenclature in the world- International Union of Pure and Applied Chemists (IUPAC) and Chemical Abstracts Service (CAS). IUPAC tends to be taught in undergraduate chemistry because it always has been and is maybe a trifle easier.
The CAS databases contain more than 200 million organic and inorganic chemical substances and about 70 million protein and nucleic acid sequences. There are two search platforms available in CAS- SciFinder and STN. STN is much more cryptic and harder to learn than SciFinder. Some say there are weaknesses in patent searching in SciFinder alone. For IP work I use SciFinder, Google Patents and the USPTO in combination. All three offer different kinds of searching capability.
Levofloxacin is a biocidal antibiotic effective against both gram-positive and gram-negative bacteria. It is an inhibitor of both DNA gyrase and topoisomerase IV enzymes which are involved in shaping the geometry of bacterial plasmids, or rings of bacterial DNA. Plasmids have to fit inside the bacterial cell wall and those that are not made compact enough are too long to allow successful formation of daughter cells in reproduction resulting in cell death. Other kinds of antibiotics are bacteriostatic and often work better in one or the other of Gram-Negative or Gram-Positive bacteria. Gram stains are effective with certain types of bacterial cell walls and not with others. The ability of a dye to stain a colony of bacteria a particular way is used to help identify bacteria.
Consider the name of Levofloxacin from IUPAC: (-)-(S)-9-fluoro-2,3-dihydro-3-methyl-10-(4-methyl-1-piperazinyl)-7-oxo-7H-pyrido[1,2,3-de]-1,4-benzoxazine-6-carboxylic acid hemihydrate. The name is a string of characters with numbers indicating attachment points. The core of the structure is a 1,4-benzoxazine ring system which is festooned with a carboxylic acid and a few other groups. The core structure was identified and numbered previously by someone according to rules. The IUPAC name also specifies that it is a hemihydrate, meaning that there is one molecule of water associated with every two (hemi) molecules of Levofloxacin. For some reason the CAS name does not include the hemihydrate in the name, probably because it was not mentioned in the composition when registered with CAS. How it is in the IUPAC name is not known to me.
More pain. The IUPAC name above indicates “(-)-(S)-“. Molecules with “handedness” are said to be chiral and are not superimposable with their mirror images, similar to a right-hand being shape-incompatible with a left glove. These molecules can be prepared as individuals of single handedness or all of the way to a 50:50 mixture of left and right-handed. A 50:50 mixture of left and right-handed is called a “racemate” (RASS eh mate). Each handedness version is a type of isomer called an “enantiomer“. A substance consisting of a pure enantiomer is said to be “enantiomerically pure.”
Isolated enantiomers have the ability to rotate plane polarized light as measured by a polarimeter. Plane polarized light is a light beam where the electric field vectors of the electromagnetic radiation are all vibrating in a single plane. Obviously the magnetic vectors are polarized as well, but it is the electric field that is usually mentioned. The angle of the oscillating ray’s electric fields along the axis can be tilted one way or the other depending on the interaction with matter. Reflected light and skyglow are polarized as well. Molecules with handedness rotate the vibrational plane and by an angle dependent on the light frequency and the amount of chiral mass traveled through. Light that is rotated counterclockwise, or levorotary, has a (-) sign and signified with an “l” and light that is rotated clockwise is dextrorotary and has a (+) sign and signified with a “d.” If a molecule rotates plane polarized light, the substance is said to be “optically active.” The amount of rotation is dependent on the light frequency, frequently the sodium D line (actually a close doublet) which is often used as the standard source for this. Mercury lines, e.g., 354 nm, can be used if the D line results in a low measured rotation. Substances that do not rotate plane polarized light are often designated “dl” as an abbreviation for racemic.
D-Glucose, or dextrose, solutions rotate plane polarized light in the clockwise, dextrorotary direction, thus the “D” in the name.
Commercial L-lactic acid derived from fermentation is “L” for levorotary. This enantiomerically enriched lactic acid is used to make the lactide monomer for poly(lactic acid), PLA. Only the lactide dimer from L-lactic acid gives the desired PLA isomer. The racemic form of lactic acid is not useful for PLA due to undesirable physical properties in the polymer.
A ratio can be taken from an experimental sample that may range from 50:50 racemate to 100 % of a single enantiomer to give the optical purity of the chiral material, representing the proportion of pure enantiomer. Often the measure % ee, or percent enantiomeric excess is used to describe enantiomeric purity. A 95:5 mixture of enantiomers would have a 90 % excess enantiomeric of one enantiomer. Chemical synthesis of 99 % ee can be quite difficult.
A racemate does not have a net rotation of plane polarized light. The (-) sign represents the “levo” part of the levofloxacin, referring to counterclockwise rotation of plane polarized light. Prior to the appearance of reliable analytical methods for the determination of enantiomeric purity, polarimetry and optical rotation were the method of choice. Today, Gas Chromatography (GC) and High Performance Liquid Chromatography (HPLC) columns and chiral shift reagents for 1H-NMR that can provide baseline separation of enantiomers.
The (S) character in the name indicates the handedness of a molecule as determined by standard selection rules defined by an organization for assigning absolute configuration. “S” stands for the Latin word “sinister” meaning left-handed. There is no simple calculation to go between absolute configuration and sign. The (-) sign can indicate which particular enantiomer is under consideration with an easy measurement if it has been previously correlated. (-)-(R) and (+)-(S) enantiomers can and do occur. The “(S)” defines only the precise configuration of atoms about an asymmetrically situated atom in a molecule based on a few simple rules. The mirror image of (-)-(S)- would be (+)-(R)-, “R” for rectus meaning right-handed in Latin.
The first task in assigning a name to a molecule is to determine the “core” structure. This is the basis of the name. Your molecule will be a variety of “the core structure.” This is not so easy because IUPAC or CAS will have already done this and your choice may or may not match. Referring to the CAS name below, you can see that some structural fragments end in “-yl,” “-ic” or “-o”. These signal that the fragments are not the core structure, they are attachments. The core structure onto which everything else is attached is the “1,4-benzoxazine”. It is a standalone chemical name which may be modified. This is a very obscure fact that most won’t know, but the “-ine” suffix indicates that the core structure is an amine, full stop. Other nitrogen indicators like azo, aza, amino, ammonium, nitro, azido, etc, suggest a nitrogen group attachment to something else.
Does it help to have a college degree in chemistry to know this stuff? Sorry but yes. In the set of all worldly knowledge, this is pretty obscure.
The CAS name for levofloxacin is 7H-Pyrido[1,2,3-de]-1,4-benzoxazine-6-carboxylic acid, 9-fluoro-2,3-dihydro-3-methyl-10-(4-methyl-1-piperazinyl)-7-oxo-, (3S)-. The core structure seems to be the 1,4-benzoxazine. CAS has a ring-system handbook that defines and numbers all of the known ring systems. The significance of CAS is that they assign and maintains the official CAS registry number, CASRN, which is depended upon world-wide for the exact composition and connectivity and geometry of substances. There is a very extensive rule book that rigidly defines a chemical name with rooms of CAS experts sitting in a building in Columbus, OH, to assign these names. For levofloxacin the CASRN is 100986-85-4. Today, CASRNs are usually directly searchable on Google. The final digit “4” is a check digit for error entry detection.
Yet more pain. The more formal official CAS name, however, does not indicate the direction of rotation of plane polarized light. I suppose this is considered experimental data not needed in the name. The CAS nomenclature only shows “(3S)-” in the name, indicating the absolute “S” configuration at position 3 of the molecule. The business of handedness or shape in 3-space of a molecule is called “stereochemistry” and arises in several ways. The rules for assigning the absolute configurations of R or S enantiomers may depend on the features of the molecule.
This business of molecular handedness is mostly an issue for biochemistry and pharmaceuticals. A great many- most?- biomolecules have handedness themselves and are therefore subject to interactions with other biomolecules or drugs that depend on the precise shapes for their interactions. This is very important for the interaction between molecules like an enzyme and substrate or ligand.
In the absence of other chiral molecules, two enantiomers will have the same chemical properties when individually pure. However, a racemate consists of a pair of enantiomers. The interactions between R and R or S and S enantiomers, will be different than the interactions between a racemic mixture of R and S or S and R enantiomers. If there is more than one chiral feature in a molecule- say two- then the molecule could be R, R or R, S, or S, S, or S, R. This gives two pairs of enantiomers, each called a “diastereomer.” For instance, one substance with R, S and another with R,R will be substances chemically and physically different called diastereomers. The presence of a diastereomer in an enantiomeric drug product would likely be deemed a contaminant and removed.

A druggable disease-state is one that can be positively influenced by a drug molecule. This commonly involves the drug molecule docking with an enzyme to activate it or deactivate it. These enzymes are very large diastereomers having many chiral atoms giving them complex shapes that can result if the formation of a pocket in the protein structure called the “active site.” This active site has very particular shape and charge features provided by the chiral amino acid chain of the protein. An active site will have a shape that is compatible with the close fitting of a drug or other molecule similar to a hand in a glove. Many of these active sites bind the shape and charge of one enantiomer of a drug molecule more effectively than the other for a better fit. The drug, or substrate, may just sit there and block the action of an enzyme, shutting it down or activate it continuously. Other active sites may bind a drug and change the shape of the enzyme causing the enzyme to speed up or slow down for a throttling or accelerating effect elsewhere on the enzyme. This is called the allosteric effect.
So, you may be asking- big deal, what does it matter? In the world of pharmaceuticals, many drug substances can exist as single enantiomers, racemates or diastereomers. Racemates may be easiest to manufacture, but very often one of the enantiomers is more biologically active than the other. In fact, one enantiomer may be disastrously harmful. The classic example is Thalidomide. The S form caused birth defects and the R form did not. Pure R enantiomer was safe from teratogenicity but a racemic mixture of R and S was not.
Conclusion. A superficial look at a chemical name opens up insights into the chemical nature of a substance. What makes each chemical substance unique is their distribution of charge in 3-dimensions. The distribution is affected by the types of the atoms present, geometric features of the 3-dimensional shape and the ability of the system to allow charge to accumulate in particular places of the molecule. These attributes mentioned also set up the type and vigor of reactivity the molecule will display.
So, I get an email from Amazon promoting its “Decarboxylator” product. The Amazon page shows a picture describing the device and shows a picture of someone loading it with spinach leaves. The title of the page says “Decarboxylator Machine to Make Butter, Oil, and More“. A link to ecru, the seller, extols the virtue of herb consumption for greater wellness. The device obviously is just a heated container with digital thermometer and temperature setpoint adjustment.

Why bring this up? This was sent to me as an Amazon customer, but I also happen to be an organic chemist who knows about decarboxylation generally. Or, just maybe they know that already?? What on Earth is retail decarboxylation about I wondered. Well, a simple Google search immediately turns up the answer. Processing weed for use in edibles. The silly allusions to vegetable processing is just a ruse.
Apparently, there are two isomers of tetrahydrocannabinolic acid, THCA. They are THCA-A and THCA-B. THCA-A is present is large quantities in unprocessed marijuana. THCA-A is the direct precursor of THC in the plant. When you smoke weed or bake it into brownies the burning or baking process decarboxylates THCA-A giving the psychoactive product, THC. However, when you extract weed with a solvent without heating, the decarboxylation is very slow and affords reduced potency. Weed for edibles must be heat treated to induce decarboxylation for maximum potency. The Wikipedia page on tetrahydrocannabinolic acid is very informative. The THCA-A precursor has its own pharmacological effects which is interesting in itself, but that is for another day.
This handy-dandy whizbang device does the deed for home producers of edibles. Ain’t it grand?

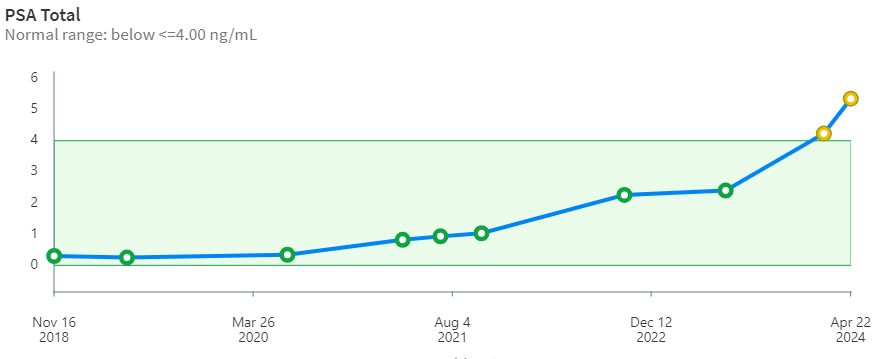

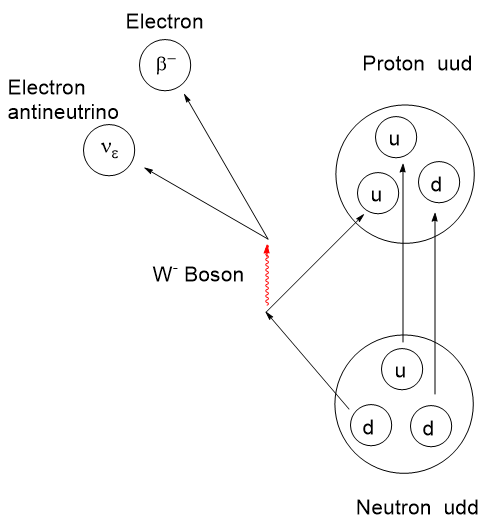
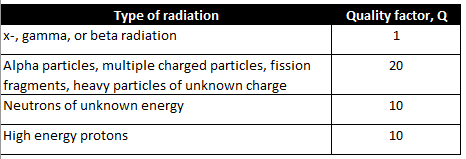

















Recent Comments